Welcome to the award-winning BU Research Blog!
Our research shapes and changes the world around us, providing solutions to real-world problems and informing the education we deliver.
Click here to view the full blog, or browse the categories below and with the menu above.
BU staff can login below:
Don’t miss a post!
Subscribe for the BU Research Digest, delivered freshly every day.
Funding opportunities
View current funding opportunities »- MSCA Postdoctoral Fellowships 2024
- BU ECRN Funding call NOW OPEN – Deadline 26th April
- BU ECRN Funding call NOW OPEN
- Q&A event: applying for the ESRC Festival of Social Science
- New seed fund for public engagement with research: apply now
- Open Call for HEIF Knowledge Exchange Project Applications 2024
BU research
 Methods or Methodology?Yesterday our latest methodological paper ‘Methods or Methodology: Terms That Are Too Often Confused’ appeared online. [1] We recently published... Read more »
Methods or Methodology?Yesterday our latest methodological paper ‘Methods or Methodology: Terms That Are Too Often Confused’ appeared online. [1] We recently published... Read more » SUNRISE sustainability conference – launch Wed 24th AprilSUNRISE (Supporting University Network for Research in Sustainability Engagement) is a British Council funded project, managed by BU in collaboration... Read more »
SUNRISE sustainability conference – launch Wed 24th AprilSUNRISE (Supporting University Network for Research in Sustainability Engagement) is a British Council funded project, managed by BU in collaboration... Read more » Upcoming opportunities for PGRs – collaborate externallySharing on behalf of Professor Fiona Cownie: We have two opportunities for PGRs to collaborate externally. On Wed 24 April we are... Read more »
Upcoming opportunities for PGRs – collaborate externallySharing on behalf of Professor Fiona Cownie: We have two opportunities for PGRs to collaborate externally. On Wed 24 April we are... Read more » BU involved in new MRF dissemination grantBournemouth University of part of a research consortium that has recently been awarded a Dissemination Award from the Medical Research... Read more »
BU involved in new MRF dissemination grantBournemouth University of part of a research consortium that has recently been awarded a Dissemination Award from the Medical Research... Read more » New COVID-19 publicationThis week the Asia Pacific Journal of Public Health (APJPH) accepted our latest paper from our research on the impact of... Read more »
New COVID-19 publicationThis week the Asia Pacific Journal of Public Health (APJPH) accepted our latest paper from our research on the impact of... Read more » Funding to compare imaging modalities for liver cancer detectionBournemouth University researchers at the Institute of Medical Imaging and Visualisation (IMIV) will contribute to a new national study evaluating... Read more »
Funding to compare imaging modalities for liver cancer detectionBournemouth University researchers at the Institute of Medical Imaging and Visualisation (IMIV) will contribute to a new national study evaluating... Read more »
international
PG research
Research lifecycle
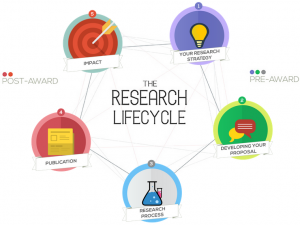 Our Research Lifecycle diagram is a jazzy interactive part of the BU Research Blog that shows the support and initiatives that are available to staff and students at each stage of the research lifecycle.
Our Research Lifecycle diagram is a jazzy interactive part of the BU Research Blog that shows the support and initiatives that are available to staff and students at each stage of the research lifecycle.











