
BU’s Knowledge, Exchange and Impact Team hosted a sold out Public Lecture Day at the Executive Business Centre back in April. A varied programme of lectures was presented to members of the public along with invited members of local University of the third age groups which included: Bournemouth, Poole, Christchurch and Ferndown.
Staying Active and Independent for Longer (SAIL)
After lunch, the afternoon of lectures began with Ann Hemingway, Professor of Public Health & Well-Being from the Faculty of Health & Social Sciences and Adele Ladkin, Professor of Tourism Employment from the Faculty of Management, who presented an overview of the Staying Active and Independent for Longer (SAIL) project.
The SAIL project, which runs until 2020 is funded by the European Commission and aims to identify new and sustainable partnership opportunities between areas such as leisure, tourism and health to help ageing people be more active and remain independent for longer.
 The SAIL research team are working with local authorities, businesses, charities and older people to learn from experiences and to try out different strategies and is comprised of team members from the Netherlands, Belgium, France and the UK.
The SAIL research team are working with local authorities, businesses, charities and older people to learn from experiences and to try out different strategies and is comprised of team members from the Netherlands, Belgium, France and the UK.
Unlike other SAIL partners, the team at Bournemouth University are not delivering initiatives to older people. Instead, they are drawing together the learning from all initiatives to manage a feasibility study for this international project. This study will, assess contribution to healthy ageing or viability of business and service models, to pave the way for wider-scale implementation.
The BU team are using the Expertise Management Methodology (EMM) which has been developed to explore and access complex real-life initiatives.
Back Pain: Another way to look at it
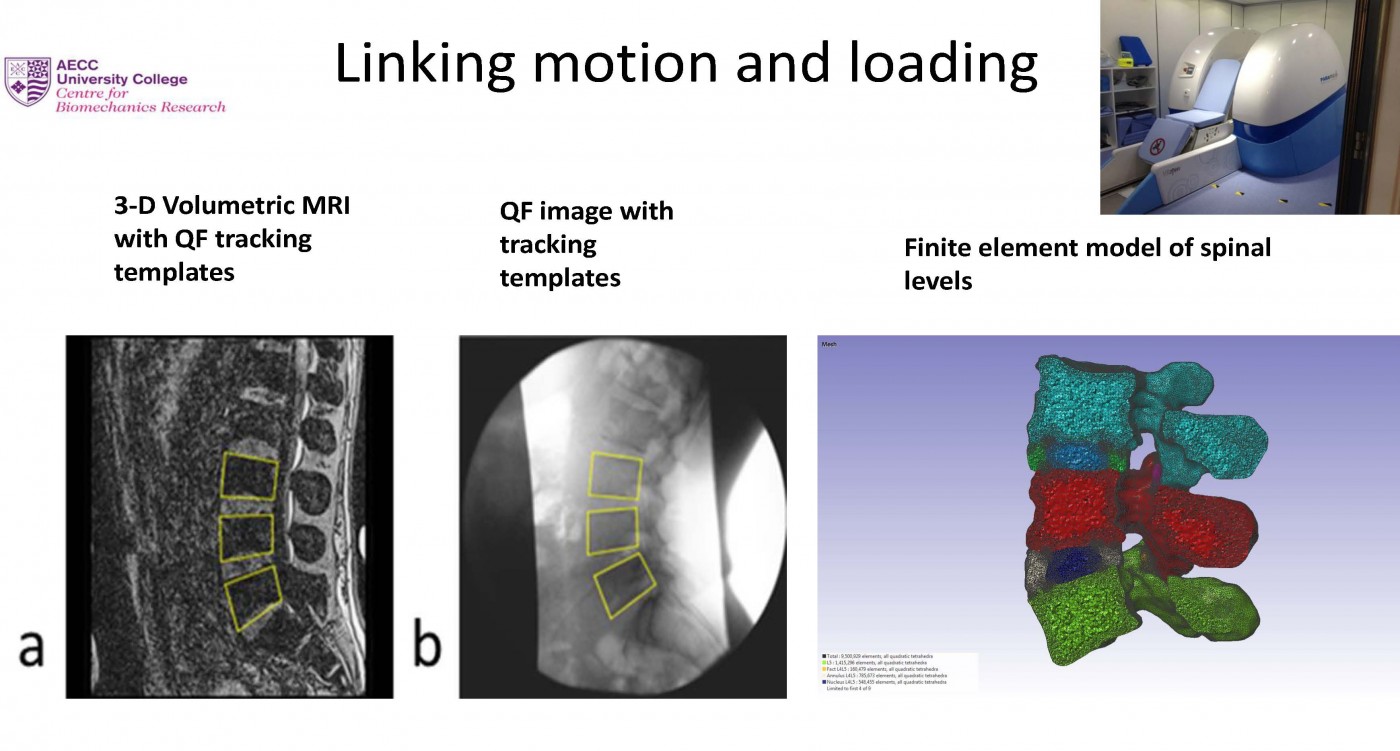
Next on the programme was Alan Breen, Professor of Musculoskeletal Research in the Faculty of Science and Technology at BU and Director of the Centre for Biomechanics Research at the AECC University College.

Professor Breen introduced his research – Back Pain: Another way of looking at it. Lower back pain is the largest cause of days lost to disability in the world and is felt at some point in the lives of around 80% of us. While the statistics are staggering, they are getting worse, with the majority of cases having no diagnosis, let alone a cure. Why is this condition so resistant to the personalised medical advances to which so many other disorders are gradually surrendering?
Alan’s talk compared how we used to understand back pain in Victorian times, when manual handling was unregulated and injuries were rife, with how we understand it now, when we know much more about the risks of becoming a chronic sufferer. In the years between the two, (in the 1990’s) Alan served on the main research review groups that drove the ‘Back Pain Revolution’. This acknowledges that whether we get better or not is not just a physical matter. However, researchers have recently realised that we don’t really know enough about the mechanisms of back pain to gain control over it in individuals. Overcoming this is the new way of looking at it.
The research being conducted in Alan’s Centre focuses on these mechanisms. Using motion X-rays (fluoroscopy), he and his colleagues have been able to bring forward a new technology to measure the mechanics of the spine at individual segments to identify ‘markers’ that characterise the functional changes that happen with back pain. Alan presented examples of how this has led to improvement with relatively simple lifestyle changes.
From Alan’s point of view, “Giving a talk of this kind to a more mature audience, many of whom had suffered with back pain for many years was rewarding, because of my interest in the early prevention of chronic back pain.”
 The Hyksos Enigma: Researching the 15th Dynasty of Ancient Egypt
The Hyksos Enigma: Researching the 15th Dynasty of Ancient Egypt
After a short break, the afternoon continued with Dr Chris Stantis, a postdoctoral researcher at BU. Her role as a bio archaeologist involves studying human remains (skeletons, cremations, mummies) from archaeological sites. By studying the people of the past, we can understand who they were, how they lived, and how they died.

At #BUPLD2019 Dr Stantis presented some of her research on ancient Egypt. The team she is working with are examining the skeletal remains of people and animals buried in cemeteries at the site of Tell el-Dab’a in the northeastern Nile Delta. Many of these people would have lived in the city when it was known as Avaris and was the capital of the 15th Dynasty. The 15th Dynasty is also known as the Hyksos period, and is the first time foreign rulers controlled Egypt. The Hyksos rulers had long lasting impacts on Egyptian culture, but no one knows for sure where the Hyksos came from or who they were. Quite the enigma!
The team are doing some amazing things at BU, studying topics such as ancestry and disease.
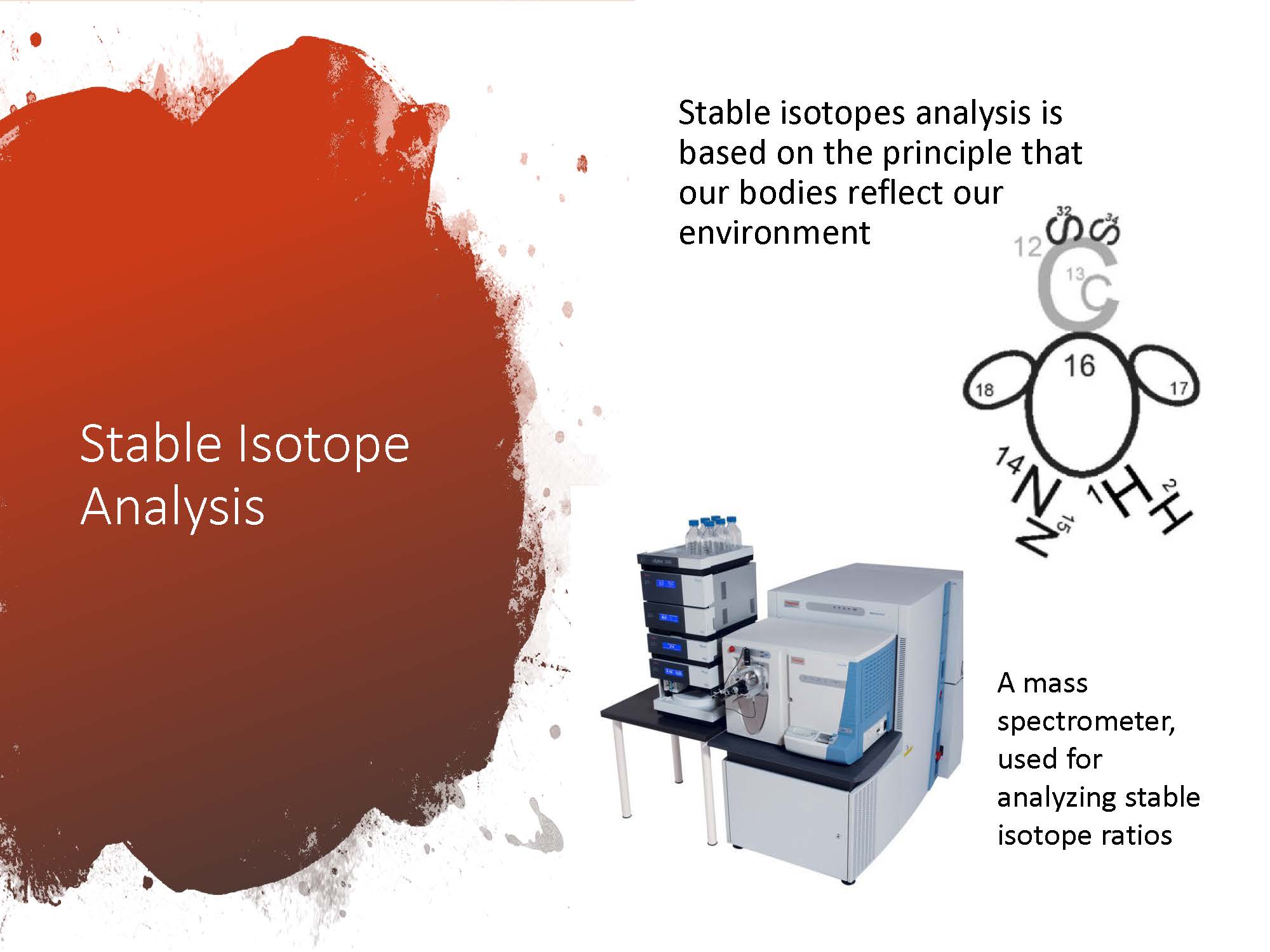
Dr Stantis is focusing on chemical analysis to understand where these people grew up, in order to try and find the origin of the Hyksos rulers. The people and culture they descended from is still a mystery, but with the help of archaeological chemistry she hopes to provide some clues.
Dr Stantis said “I loved presenting some of my initial findings at #BUPLD2019. The audience was engaged, they asked really good questions, and of course there were free sandwiches and coffee”
The effect of emotion on cognition: What can we learn from those with obsessive-compulsive disorders?
The afternoon of lectures was closed by Dr Ellen Seiss, Senior Lecturer in Psychology and Head of the SC2AN Neuroscience Research Group within the Department of Psychology.
Ellen is conducting research in cognitive and clinical neuroscience using behavioural and EEG methods, her current areas of interest are the cognitive deficits in people with OCD, emotion recognition in VR settings, as well as the link between food choice and intake and cognition.
Dr Seiss discussed the effects of emotion on cognition and what we can learn from those with obsessive compulsive disorders and explained that our emotions influence a lot of our everyday cognitions, for example how we tend to certain situations, how we memorise information and how we make decisions. This emotional effect is more pronounced in people living with anxiety disorders and obsessive-compulsive disorders. Those affected often try to avoid ‘harmful’ events with ritualised behaviours; such as checking doors, lights, or extreme cleanliness rituals.

Dr Seiss concluded that the interaction between emotions and processes are very complex in people with OCD. For example; selective attention, memory and memory confidence, decision making in uncertain environments.
Ellen highlighted the relevance of this research; If we know how people with OCD process information to memorise it and to make decisions, and what the underlying neurological changes are, we might be able to use this to adjust our OCD models and treatment options.
 Bournemouth University’s Public Lecture series aims to offer BU academics the opportunity to share some of the fantastic research they’ve been working on with members of the public. Allowing a diverse and enthusiastic audience to ask lots of questions and engage in interesting discussion about the research taking place here at Bournemouth University.
Bournemouth University’s Public Lecture series aims to offer BU academics the opportunity to share some of the fantastic research they’ve been working on with members of the public. Allowing a diverse and enthusiastic audience to ask lots of questions and engage in interesting discussion about the research taking place here at Bournemouth University.
The varied programme was well received with some audience members commenting;
“The event helped with the concept of bringing people, mainly older, together. We met many people from our local community we did not know”
“Very enjoyable to hear up to date ideas and research”
” Learning about subjects I knew nothing about”
“Interesting lectures related to the real world”
If you missed the Public Lecture Day and would like a copy of the presentations or require more information about this event please contact the Public Engagement Team; publicengagement@bournemouth.ac.uk



 The SAIL research team are working with local authorities, businesses, charities and older people to learn from experiences and to try out different strategies and is comprised of team members from the Netherlands, Belgium, France and the UK.
The SAIL research team are working with local authorities, businesses, charities and older people to learn from experiences and to try out different strategies and is comprised of team members from the Netherlands, Belgium, France and the UK.
 The Hyksos Enigma: Researching the 15th Dynasty of Ancient Egypt
The Hyksos Enigma: Researching the 15th Dynasty of Ancient Egypt


 Bournemouth University’s Public Lecture series aims to offer BU academics the opportunity to share some of the fantastic research they’ve been working on with members of the public. Allowing a diverse and enthusiastic audience to ask lots of questions and engage in interesting discussion about the research taking place here at Bournemouth University.
Bournemouth University’s Public Lecture series aims to offer BU academics the opportunity to share some of the fantastic research they’ve been working on with members of the public. Allowing a diverse and enthusiastic audience to ask lots of questions and engage in interesting discussion about the research taking place here at Bournemouth University.





 Anya’s Café Scientifique talk covered the rise and development of British seaside piers and considered their changing roles and purpose during the last 200 years. The current challenges faced by seaside piers were also examined, including the threats facing these structures in an era of climate change, rising sea levels, and storm surges. The issue of funding and costs of piers was considered and the question of ‘who pays’ for these iconic seaside heritage assets was discussed.
Anya’s Café Scientifique talk covered the rise and development of British seaside piers and considered their changing roles and purpose during the last 200 years. The current challenges faced by seaside piers were also examined, including the threats facing these structures in an era of climate change, rising sea levels, and storm surges. The issue of funding and costs of piers was considered and the question of ‘who pays’ for these iconic seaside heritage assets was discussed.

 Anya said ‘the experience of presenting at Café Scientifique was enjoyable and enlightening. It was really insightful to hear about people’s experience of piers and how they value and engage with these structures. It was also good to see the levels of engagement and discussion on the issue of climate change and how seaside piers might be used in the not-too distant future as a source of blue energy production, meaning that the form and function of piers will continue to adapt in the 21st century’.
Anya said ‘the experience of presenting at Café Scientifique was enjoyable and enlightening. It was really insightful to hear about people’s experience of piers and how they value and engage with these structures. It was also good to see the levels of engagement and discussion on the issue of climate change and how seaside piers might be used in the not-too distant future as a source of blue energy production, meaning that the form and function of piers will continue to adapt in the 21st century’.






 The article titled “The effects of 8 weeks of inspiratory muscle training on the balance of healthy older adults: a randomized, double-blind, placebo-controlled study” has been published by Physiological Reports.
The article titled “The effects of 8 weeks of inspiratory muscle training on the balance of healthy older adults: a randomized, double-blind, placebo-controlled study” has been published by Physiological Reports. Alzheimer’s Research UK have opened applications to the Inspire Fund, their new public engagement grant scheme, to support more people to engage the public with dementia and research into the condition.
Alzheimer’s Research UK have opened applications to the Inspire Fund, their new public engagement grant scheme, to support more people to engage the public with dementia and research into the condition.










 Upcoming opportunities for PGRs – collaborate externally
Upcoming opportunities for PGRs – collaborate externally BU involved in new MRF dissemination grant
BU involved in new MRF dissemination grant New COVID-19 publication
New COVID-19 publication MSCA Postdoctoral Fellowships 2024
MSCA Postdoctoral Fellowships 2024 Horizon Europe News – December 2023
Horizon Europe News – December 2023