Welcome to the award-winning BU Research Blog!
Our research shapes and changes the world around us, providing solutions to real-world problems and informing the education we deliver.
Click here to view the full blog, or browse the categories below and with the menu above.
BU staff can login below:
Don’t miss a post!
Subscribe for the BU Research Digest, delivered freshly every day.
BU research
 AI and Academic Identities: Navigating the FutureThe RKEDF is excited to announce this ECRN seed funded event: AI and Academic Identities: Navigating the Future 📅 Thursday,... Read more »
AI and Academic Identities: Navigating the FutureThe RKEDF is excited to announce this ECRN seed funded event: AI and Academic Identities: Navigating the Future 📅 Thursday,... Read more » PramaLife to present at the at Community Voices Webinar Wednesday 9 July, 12-1pmWe’re excited to welcome Sue Warr, Manager at PramaLife, to our July Community Voices Webinar. Sue will be sharing insights... Read more »
PramaLife to present at the at Community Voices Webinar Wednesday 9 July, 12-1pmWe’re excited to welcome Sue Warr, Manager at PramaLife, to our July Community Voices Webinar. Sue will be sharing insights... Read more » Dr. Ashraf cited on ‘Modest Fashion’ in The GuardianEarlier this month (15 June) Dr. Samreen Ashraf, was cited in the newspaper The Guardian in an interesting article with... Read more »
Dr. Ashraf cited on ‘Modest Fashion’ in The GuardianEarlier this month (15 June) Dr. Samreen Ashraf, was cited in the newspaper The Guardian in an interesting article with... Read more » NIHR-funded research launches websiteOn World Refugee Day 2025, Friday 20 June, the new Maternal and Infant Health Equity Research Centre (MIHERC) website was... Read more »
NIHR-funded research launches websiteOn World Refugee Day 2025, Friday 20 June, the new Maternal and Infant Health Equity Research Centre (MIHERC) website was... Read more » Academics write for newspaper in NepalYesterday the online newspaper Online Khabar in Nepal published an opinion piece in English written by three Bournemouth University academics working with... Read more »
Academics write for newspaper in NepalYesterday the online newspaper Online Khabar in Nepal published an opinion piece in English written by three Bournemouth University academics working with... Read more » New paper published on disability in women & girlsYesterday (25 June) the online journal PLoS One published ‘Life-time experience of violence among women and girls living with disability in... Read more »
New paper published on disability in women & girlsYesterday (25 June) the online journal PLoS One published ‘Life-time experience of violence among women and girls living with disability in... Read more »
international
PG research
Research lifecycle
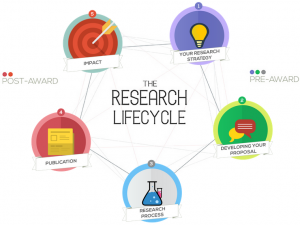 Our Research Lifecycle diagram is a jazzy interactive part of the BU Research Blog that shows the support and initiatives that are available to staff and students at each stage of the research lifecycle.
Our Research Lifecycle diagram is a jazzy interactive part of the BU Research Blog that shows the support and initiatives that are available to staff and students at each stage of the research lifecycle.











