In the UK 12-15% of pregnancies are complicated by high blood pressure. Specifically, pregnancy-induced hypertension (PIH) is high blood pressure that is diagnosed during the pregnancy and was not present before. Women with PIH normally return to having normal blood pressure within a few weeks of giving birth but during the pregnancy high blood pressure can cause many problems with women often having to spend extra time in hospital and having to be induced to give birth preterm.
Researchers at BU are addressing this issue by seeking to design a new alternative drug-free treatment for high blood pressure. Slow and deep breathing has been shown to reduce blood pressure chronically when practiced daily over a period of 6-8 weeks in non-pregnant people who have high blood pressure. The Brythm App, using a graphic designed by a BU Student Research Assistant, uses bio-feedback to dynamically reduce the user’s breathing frequency to a personalised optimum. However, despite the research showing chronic adaptations from slow and deep breathing very little is known about the acute responses to slow and deep breathing. Therefore, the BU project started by characterising the short-term physiological effects of slow and deep breathing with women of childbearing age.
With assistance from BU staff and student members, the first testing phase of the BU Brythm App was completed last year. The results show that there is an immediate physiological response in heart rate and blood pressure during just 5 minutes of slow and deep breathing. Specifically, this study allowed us to move forward in identifying the optimal breathing frequency which we believe can maximise the cardiovascular responses. Participants who took part last year will be pleased to know that future participants no longer need to undertake the inspiratory resistance protocol, where participants breathed through a Powerbreathe medic, as this was found not to elicit any additional benefit compared with the other slow and deep breathing protocols.
The second testing phase for Brythm, as part of my PhD project, is to examine the short-term responses to slow and deep breathing within a pregnant population. This study will predominantly replicate the first study protocol to investigate whether the physiological changes caused by pregnancy influence the responses to slow and deep breathing. The aim of this study is to identify the optimal breathing frequency that will be used in the final study of my PhD. The final study will be an interventional study with pregnant women who have high blood pressure. They will use the Brythm App at home on their own devices for 10 minutes every day for a period of 8 weeks.
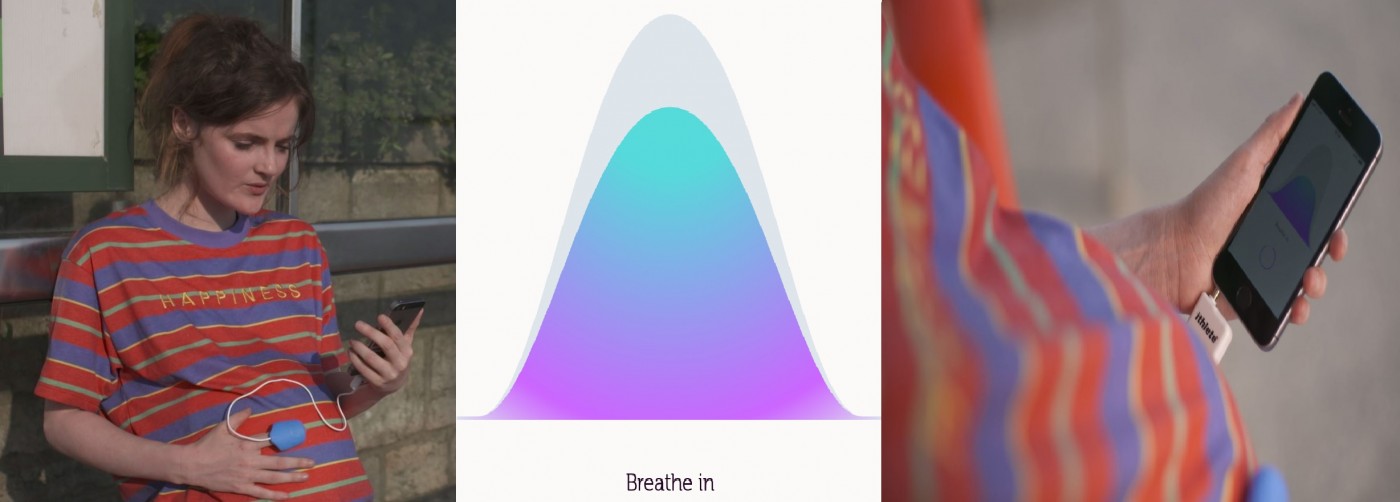
The current study will investigate the physiological responses to multiple breathing frequencies; 4, 6 and 8 breaths per minute, and a dynamic breathing frequency controlled by an in-built algorithm. This may seem a low breathing frequency but your body compensates automatically by taking deeper breaths, and some participants feel so relaxed during the protocols they often nearly fall asleep. These protocols will be compared to spontaneous normal breathing and each protocol will be 5 minutes in duration. Heart rate and blood pressure will be monitored continuously to find the optimal breathing frequency for pregnant women, which maximises the physiological responses. Participation involves a one off session which takes place at the Lansdowne Campus and lasts for approximately 90 minutes. Participants will receive a £20 Amazon voucher as a thank you for their time.
If you would like to participate, or know anyone who is currently pregnant who may want to take part, then please read the following inclusion and exclusion criteria and contact Malika Felton (mfelton@bournemouth.ac.uk & 01202 961845). More information can be found on the Brythm website (https://www.brythm.com/news/research/pregnancyresponses/).
To participate you must be:
· Over 20 weeks gestation with first pregnancy;
· Carrying a single pregnancy (not twins, triplets, etc.);
· Aged 18 or over and a non-smoker;
· Have no current diagnosis of:
o Hypertension, pregnancy-induced hypertension or preeclampsia;
o Asthma, bronchitis, COPD;
o An allergy/reaction to the gel used for ECG.
 Are you interested in testing an App developed at BU and designed to lower blood pressure?
Are you interested in testing an App developed at BU and designed to lower blood pressure? How breathing slowly can help lower blood pressure and reduce the risk of serious health conditions
How breathing slowly can help lower blood pressure and reduce the risk of serious health conditions Café Sci Tuesday 1 March: Deep breathing to lower blood pressure
Café Sci Tuesday 1 March: Deep breathing to lower blood pressure










 Dr. Ashraf cited on ‘Modest Fashion’ in The Guardian
Dr. Ashraf cited on ‘Modest Fashion’ in The Guardian NIHR-funded research launches website
NIHR-funded research launches website Academics write for newspaper in Nepal
Academics write for newspaper in Nepal MSCA Postdoctoral Fellowships 2025 Call
MSCA Postdoctoral Fellowships 2025 Call ERC Advanced Grant 2025 Webinar
ERC Advanced Grant 2025 Webinar Horizon Europe Work Programme 2025 Published
Horizon Europe Work Programme 2025 Published Horizon Europe 2025 Work Programme pre-Published
Horizon Europe 2025 Work Programme pre-Published Update on UKRO services
Update on UKRO services European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease
European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease