
RDS NEWS
NIHR Grant Applications Seminar and Support event
Our popular seminar continues online and will next take place in March 2023. Watch this space for further details.
In the meantime, you can register your interest with us and once details of the event are available, you will receive an email with a weblink to find out more.
Alternatively, you can contact our Coordinating Centre on 01392 726724 or rds.sw@nihr.ac.uk, or your local RDS office to find out more.
NIHR News
Experimental treatments for cancer receive funding boost
The NIHR is contributing £21.6 million to the network of Experimental Cancer Medicine Centres (ECMCs) as part of a £47.5 million funding package over the next 5 years. Find out more
Workshops and Events
NIHR webinar: Discover funding opportunities for qualitative researchers
7 February 2023, 10.30am – 12.30pm. ONLINE
This webinar is hosted by the Qualitative Workstream of the NIHR Methodology Incubator and the NIHR Academy. The webinar aims to support the careers of qualitative and mixed methods researchers by outlining funding opportunities for health and care research. Speakers will provide an overview of funding schemes including personal fellowships, small project grants, large research programmes and grants for methods research. Participants will hear from NIHR Senior Investigators, Directors of funding schemes as well as people who manage schemes and sit on funding committees. You will have an opportunity to ask questions to the speakers in a Q&A session at the end. Find out more.
NIHR webinar: Embedding PPIE in your research
21 February 2023, 1.00pm – 2.00pm. ONLINE
This webinar will demonstrate how to integrate PPIE into your research and focus on working with underserved groups or topics. The difficulty facing research teams, especially early career researchers, is when and how to use the public and patients.
We know that the communities mostly likely to experience health inequalities and benefit most from research are the underserved and underrepresented. The webinar will explore ways of answering the following questions:
- How would you target a group/community to participate in your research?
- What tools, techniques and partners would you enlist to make sure your target community is able to get involved in your research?
- How would you know if you got it right?
Find out more.
NCRM webinar series: Data resources for mental health and wellbeing research
6 March 2023. ONLINE
A two-part webinar series will explain how to access and use datasets on mental health and wellbeing. Organised by the Data Resources Training Network (DRTN), the free series begins on Monday, 6 March with a webinar on secondary, quantitative data. The speakers will be Sally McManus from NatCen, Eoin McElroy from Ulster University and Mark Elliot from The University of Manchester. To complement the series, NCRM has collated a suite of resources relating to mental health and wellbeing, which will be launched to coincide with the events. Find out more.
Funding Opportunities
Funding deadline calendar
For an overview of NIHR calls and ongoing funding opportunities, please see our funding deadlines webpage
Latest NIHR funding calls
Fellowship Programme
Pre-doctoral Fellowship (Round 5)
HEE-NIHR Integrated Clinical and Practitioner Academic Programme
Pre-doctoral Clinical and Practitioner Academic Fellowship (PCAF) Round 6
Health and Social Care Delivery Research (HSDR) Programme
23/8 Care (Education) and Treatment Reviews (C(E)TRs) for people with learning disabilities and/or autistic people
Local Authority Academic Fellowship Programme
Pre-doctoral Local Authority Fellowship (PLAF) Round 3
Public Health Research (PHR) Programme
23/12 Health Determinant Research Collaborations (HDRCs)
Themed call
Compound pressures
Your local branch of the NIHR RDS (Research Design Service) is based within the BU Clinical Research Unit (BUCRU) should you need help with any grant applications. We advise on all aspects of developing an application and can review application drafts as well as put them to a mock funding panel (run by RDS South West) known as Project Review Committee, which is a fantastic opportunity for researchers to obtain a critical review of a proposed grant application before this is sent to a funding body or if you’re hoping to resubmit the panel can provide some excellent tips and feedback.
Contact us as early as possible to benefit fully from the advice
Feel free to call us on 01202 961939 or send us an email.
 This week the international scientific journal Midwifery published Ms. Joanne Rack’s second paper from her PhD research. This latest paper ‘The Pregnant Pause: Engaging and Involving Public Contributors in Maternal Health Research‘ [1] appeared online two days ago. This paper focuses on Joanne’s PPI (Patient Public Involvement) in prepartion for her PhD research. The public contributors of PPI groups can include an extensive range of people, including patients, family members or carers, people from allied organisations, service users, and members of the general public who have an interest in research for other reasons. Participants bring their unique perspectives and experiences that can help to shape and inform the research process. This type of involvement ensures that maternal health research is grounded in the needs and preferences of those it aims to serve and grows a sense of ownership and investment among those who use the services but also those who provide them. Joanne stresses that PPI is an essential element for all maternal health endeavours.
This week the international scientific journal Midwifery published Ms. Joanne Rack’s second paper from her PhD research. This latest paper ‘The Pregnant Pause: Engaging and Involving Public Contributors in Maternal Health Research‘ [1] appeared online two days ago. This paper focuses on Joanne’s PPI (Patient Public Involvement) in prepartion for her PhD research. The public contributors of PPI groups can include an extensive range of people, including patients, family members or carers, people from allied organisations, service users, and members of the general public who have an interest in research for other reasons. Participants bring their unique perspectives and experiences that can help to shape and inform the research process. This type of involvement ensures that maternal health research is grounded in the needs and preferences of those it aims to serve and grows a sense of ownership and investment among those who use the services but also those who provide them. Joanne stresses that PPI is an essential element for all maternal health endeavours.
 Her PhD is supervised and supported by Profs. Vanora Hundley, Ann Luce and Edwin van Teijlingen at BU and Dr. Latha Vinayakarao in Poole Maternity Hospital. The first PhD paper with Joanne as lead author was her research protocol ‘Understanding perceptions and communication of risk in advanced maternal age: a scoping review (protocol) on women’s engagement with health care services’ published int he summer of 2024 [2].
Her PhD is supervised and supported by Profs. Vanora Hundley, Ann Luce and Edwin van Teijlingen at BU and Dr. Latha Vinayakarao in Poole Maternity Hospital. The first PhD paper with Joanne as lead author was her research protocol ‘Understanding perceptions and communication of risk in advanced maternal age: a scoping review (protocol) on women’s engagement with health care services’ published int he summer of 2024 [2]. 






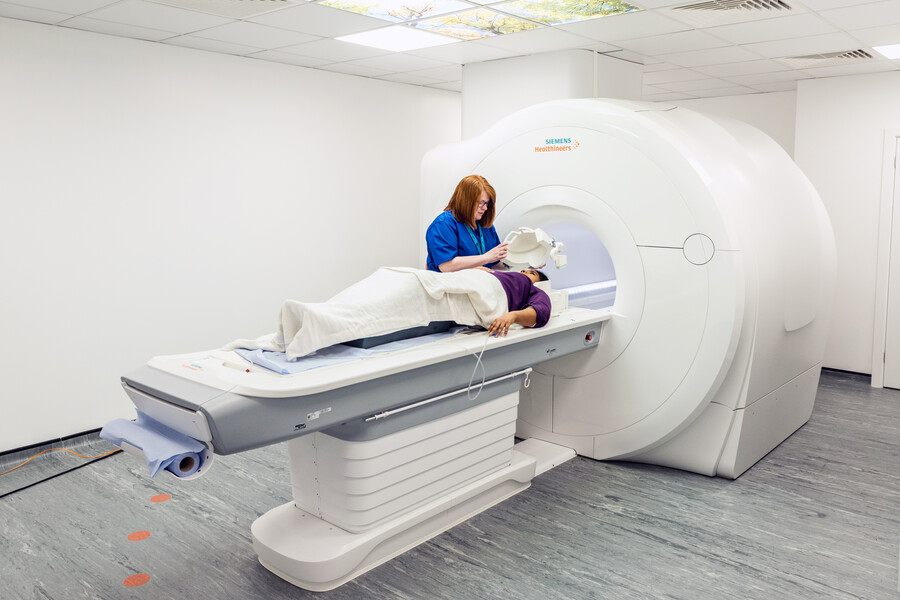 The Institute of Medical Imaging and Visualisation (IMIV) is pleased to announce the launch of the IMIV MRI Research Project Scheme 2023.
The Institute of Medical Imaging and Visualisation (IMIV) is pleased to announce the launch of the IMIV MRI Research Project Scheme 2023. Applications close on Friday 7th July 2023.
Applications close on Friday 7th July 2023. 
















 Dr. Ashraf cited on ‘Modest Fashion’ in The Guardian
Dr. Ashraf cited on ‘Modest Fashion’ in The Guardian NIHR-funded research launches website
NIHR-funded research launches website Academics write for newspaper in Nepal
Academics write for newspaper in Nepal New paper published on disability in women & girls
New paper published on disability in women & girls Global Consortium for Public Health Research 2025
Global Consortium for Public Health Research 2025 MSCA Postdoctoral Fellowships 2025 Call
MSCA Postdoctoral Fellowships 2025 Call ERC Advanced Grant 2025 Webinar
ERC Advanced Grant 2025 Webinar Horizon Europe Work Programme 2025 Published
Horizon Europe Work Programme 2025 Published Horizon Europe 2025 Work Programme pre-Published
Horizon Europe 2025 Work Programme pre-Published Update on UKRO services
Update on UKRO services European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease
European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease