Tuesday saw the annual NHS Research Ethics Committee (REC) members training day in London. The learning outcomes of the day were:
- To provide overview of the pilot work being undertaken in preparation for EU Clinical Trials Regulation
- To introduce the REWARD Alliance and,
- To consider how ethics committess can encourage researchers to engage more fully with the scientific literature both before and after studies are conducted
The morning focussed on updates on ethics regulatory procedures, the EU (see link below for slides) and changes in the Data Protection Act (but not the law of confidentiality) that have implications beyond healthcare research. There is also movement for a Public Involvement in Ethical Review (PIER) service, as well as adopting ‘e-consent’ for participation in health research.
EU Regulation_UK Research Ethics Service
The afternoon focussed on the REWARD Alliance and how ethics committees (and researchers) can help reduce waste in research. This group was established to promote a series of articles on research published in early 2014 in The Lancet.
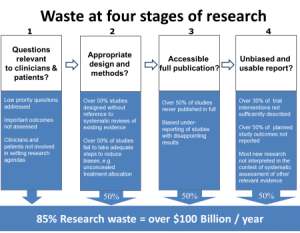
Figure: Stages of waste in producing and reporting of research evidence (Chalmers & Glasziou, The Lancet 2009).

As a researcher and ethical reviewer, the day was insightful, interesting and relevant. Knowledge of the REWARD Alliance, particularly how researchers should diligently plan and prepare projects with clear pathways to dissemination. Although publishing demands differ between academia and industry (including pharmaceutical companies), all research should be designed fom the outset with clear outputs to communicate the findings.
If you would like further information from the day, send me an email.
James
 Meet members of the Research Ethics Panels
Meet members of the Research Ethics Panels PGR Member Required – University Research Ethics Committee
PGR Member Required – University Research Ethics Committee Meet members of the Research Ethics Panels
Meet members of the Research Ethics Panels New research ethics paper Faculty of Health & Social Sciences
New research ethics paper Faculty of Health & Social Sciences










 BU attendance at third annual GCPHR meeting in June
BU attendance at third annual GCPHR meeting in June Interactive Tangible and Intangible Heritage Applications – BU student work featured in new book chapter
Interactive Tangible and Intangible Heritage Applications – BU student work featured in new book chapter Second NIHR MIHERC meeting in Bournemouth this week
Second NIHR MIHERC meeting in Bournemouth this week MSCA Postdoctoral Fellowships 2025 Call
MSCA Postdoctoral Fellowships 2025 Call ERC Advanced Grant 2025 Webinar
ERC Advanced Grant 2025 Webinar Horizon Europe Work Programme 2025 Published
Horizon Europe Work Programme 2025 Published Horizon Europe 2025 Work Programme pre-Published
Horizon Europe 2025 Work Programme pre-Published Update on UKRO services
Update on UKRO services European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease
European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease