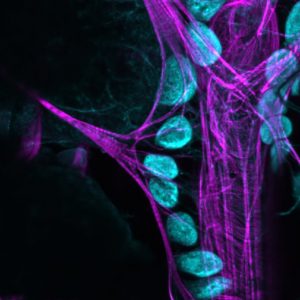
Colleagues at Cornell University and I have used the fruit fly, Drosophila to tease apart the relationship between immunity and the gut microbiome. The work (which took six years to complete) is to be published in Immunity (impact factor 20 for the ‘metricists’ out there) and has major significance because it starts to explain how the human immune response ‘tolerates’ the billions of ‘good’ bacteria in our body.
Many animals carry billions of bacteria in their intestines which are critical for the digestion of ingested foods. This poses a problem for immune cells because signs of the bacteria regularly end up outside the gut and in circulation. Normally, bacterial signals would elicit a powerful immune system but it would be bad news if the gut microbiome was targeted for destruction by immune cells. How this cordial relationship is maintained is therefore of major interest to immunologists and medical science because it has implications for how we understand inflammatory diseases.
We show for the first time that cells called nephrocytes remove bacterial signals (proteoglycans that make bacterial cell walls) from circulation and that this dampens immune responses. Disruption of this removal system causes immune cells to be over-active – a state not unlike chronic inflammation.
I’m duty bound as a basic scientist to make the point that this work also impacts our understanding of insect ecology. Having an over-active immune system shortened the lifespan of Drosophila – an effect likely to be seen in ecologically and medically important species such as honeybees and mosquitoes. How immune responses are affected by the environment in these species is also a very hot topic of research – one that can also be modeled in Drosophila.
Best wishes,
Paul Hartley (Dept of Life and Environmental Sciences)












 Expand Your Impact: Collaboration and Networking Workshops for Researchers
Expand Your Impact: Collaboration and Networking Workshops for Researchers Visiting Prof. Sujan Marahatta presenting at BU
Visiting Prof. Sujan Marahatta presenting at BU 3C Event: Research Culture, Community & Can you Guess Who? Thursday 26 March 1-2pm
3C Event: Research Culture, Community & Can you Guess Who? Thursday 26 March 1-2pm UKCGE Recognised Research Supervision Programme: Deadline Approaching
UKCGE Recognised Research Supervision Programme: Deadline Approaching ECR Funding Open Call: Research Culture & Community Grant – Apply now
ECR Funding Open Call: Research Culture & Community Grant – Apply now ECR Funding Open Call: Research Culture & Community Grant – Application Deadline Friday 12 December
ECR Funding Open Call: Research Culture & Community Grant – Application Deadline Friday 12 December MSCA Postdoctoral Fellowships 2025 Call
MSCA Postdoctoral Fellowships 2025 Call ERC Advanced Grant 2025 Webinar
ERC Advanced Grant 2025 Webinar Update on UKRO services
Update on UKRO services European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease
European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease