Every beat of the heart is finely tuned to eject a certain amount of blood. As we exercise, more blood flows into the heart, the cardiac muscle stretches and this leads to an increased force of contraction. Known as the Frank-Starling law, it is one of the most important aspects of human cardiac physiology but the molecular mechanisms are not entirely understood.
We do know that increases to the calcium levels in the heart cells (cardiomyocytes) support stronger contractions (anyone remember the ‘sliding ratchet model’ from GCSE biology!?) but how this calcium is regulated by stretch is not fully understood. What my colleagues and I have established (to be published in Frontiers of Physiology) is that a ‘mechanosensitive’ protein known as Piezo helps increase calcium when the cardiomyocytes are stretched. A lot of this work was done at BU’s Drosophila (fruit fly) genetics facility in Dorset House, using physiological tests of heart function in flies without the Piezo protein. When stretched, normal hearts respond by releasing more calcium and they continue to beat. In Piezo mutants, there’s no increase in calcium and the hearts often stop beating.
This is an important observation that contributes to our fundamental understanding of cardiac physiology and points to Piezo as a protein of considerable interest when considering the underlying causes of cardiac dysfunction in disease and ageing.
Paul Hartley.
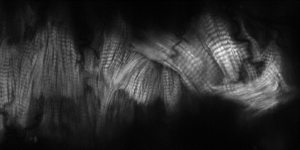
(The image shows the contractile protein ‘scaffold’ within an insect heart)











 Dr. Ashraf cited on ‘Modest Fashion’ in The Guardian
Dr. Ashraf cited on ‘Modest Fashion’ in The Guardian NIHR-funded research launches website
NIHR-funded research launches website Academics write for newspaper in Nepal
Academics write for newspaper in Nepal New paper published on disability in women & girls
New paper published on disability in women & girls Global Consortium for Public Health Research 2025
Global Consortium for Public Health Research 2025 MSCA Postdoctoral Fellowships 2025 Call
MSCA Postdoctoral Fellowships 2025 Call ERC Advanced Grant 2025 Webinar
ERC Advanced Grant 2025 Webinar Horizon Europe Work Programme 2025 Published
Horizon Europe Work Programme 2025 Published Horizon Europe 2025 Work Programme pre-Published
Horizon Europe 2025 Work Programme pre-Published Update on UKRO services
Update on UKRO services European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease
European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease