 On World Refugee Day 2025, Friday 20 June, the new Maternal and Infant Health Equity Research Centre (MIHERC) website was launched. MIHERC is a hub for research, collaboration and action on maternal and infant health equity. MIHERC) is a collaborative effort between Sheffield Hallam University, Bournemouth University and City of Doncaster Council working to reduce health inequalities for mothers and babies. This year’s World Refugee Day’s theme, hashtagSolidarity, reflects MIHERC’s mission to stand with all mothers and babies – especially those facing health and social inequalities or barriers to care.
On World Refugee Day 2025, Friday 20 June, the new Maternal and Infant Health Equity Research Centre (MIHERC) website was launched. MIHERC is a hub for research, collaboration and action on maternal and infant health equity. MIHERC) is a collaborative effort between Sheffield Hallam University, Bournemouth University and City of Doncaster Council working to reduce health inequalities for mothers and babies. This year’s World Refugee Day’s theme, hashtagSolidarity, reflects MIHERC’s mission to stand with all mothers and babies – especially those facing health and social inequalities or barriers to care.
Category / Research communication
New paper published on disability in women & girls
Yesterday (25 June) the online journal PLoS One published ‘Life-time experience of violence among women and girls living with disability in Nepal‘ our latest study on disability in Nepal [1]. This cross-sectional study was conducted in 28 municipalities representing all seven provinces as well as all three ecological regions of Nepal. A total of 1,294 women and girls with disability aged 15–59 years participated in 2021. We trained local enumerators using the KoBo application on smartphones or tablets. Both written and oral informed consent was sought from all participants. Cross-tabulations were performed in STATA 18 to determine the distribution of the prevalence of violence. Also, bivariable and multivariable logistic regression models were fitted to establish association between the participants’ characteristics and odds of experiencing violence.
Overall, 457 (35.32%) women living with disabilities had ever experienced violence at a point in their lifetime. Psychological/emotional violence was the most prevalent violence (74.40%) followed by physical violence (31.07%) and denial of services (28.67%). Age was positively associated with the likelihood of experiencing violence. Women belonging to the Brahman/Chhetri ethnic group had reduced odds of violence [AOR = 0.56; 95%CI: 0.37–0.85] compared to Hill Dalits. Divorced or separated women showed a markedly higher likelihood of experiencing violence [AOR = 6.69; 95%CI: 2.31–19.40] compared to currently married women. Participants who had not witnessed violence against other women exhibited significantly higher odds of experiencing violence [AOR = 1.86; 95%CI: 1.20–2.89]. Women living in the Koshi province [AOR = 4.04; 95%CI: 2.54–6.42], Madhesh province [AOR = 2.16; 95%CI: 1.15–4.08] and Bagmati province [AOR = 2.21; 95%CI: 1.41–3.46] reported significantly higher odds of experiencing violence compared to those in Karnali.
The paper concludes oncludes that age, ethnicity, marital status, and provincial residence are significant predictors of violence among women and girls living with disability in Nepal. Interventions aimed at addressing violence against women living with disability in Nepal must prioritize older women and those who were previously married. Also, policy-makers may want to consider giving priority must be given to those provinces where the prevalence and risk of experiencing violence is highest.
The study was funded The United Nations Women Trust Fund, and the paper is freely available in the Open Access journal. We previously published on research into disability in Nepal in 2023 [2].

Prof. Edwin van Teijlingen
Centre for Midwifery & Women’s Health
Visiting Faculty, Centre for Disability Studies, Mahatma Gandhi University, Kerala, India.
References:
- Simkhada P, Basnet S, Sharma S, van Teijlingen E, Wasti SP, Dahal T, et al. (2025) Life-time experience of violence among women and girls living with disability in Nepal. PLoS One 20(6): e0326659. https://doi.org/10.1371/journal.pone.0326659.
- Simkhada, P, Shyangdan, D, van Teijlingen E, Kadel, S, Stephen, J., Gurung, T. (2013) Women’s Knowledge & Attitude towards Disability in Rural Nepal. Disability & Rehabilitation 35(7): 606-13. http://informahealthcare.com/doi/abs/10.3109/09638288.2012.702847
Conversation article: Five ways to keep teenagers safe by the water
Dr Jill Nash writes for The Conversation about the need to educate young people about the risks of drowning, and shares her advice for helping to keep them safe…
Five ways to keep teenagers safe by the water

Jill Nash, Bournemouth University
As temperatures soar around the UK and Ireland due to climate change, warnings about the dangers of drowning are being issued and one Labour MP is calling for water safety lessons to be made compulsory in schools.
Teaching children to swim is essential, but it’s not enough to save them from drowning. Water safety is about judgement, impulse control, peer influence and understanding your limits. Peer pressure, social situations and a false sense of confidence can all put young people in danger.
My research highlights how we’re not talking enough to young people, especially teenage boys, about the emotional and cognitive risks of making decisions around water. The National Water Safety Forum reports that young males aged between 10-19 are one of the highest groups at risk from drowning, as they assert their independence and test personal boundaries.
Drowning happens quickly, often without adults watching, when kids are hanging out by rivers and lakes, tombstoning off bridges, or misjudging their abilities when trying to impress friends.
Get your news from actual experts, straight to your inbox. Sign up to our daily newsletter to receive all The Conversation UK’s latest coverage of news and research, from politics and business to the arts and sciences.
Leading water safety organisations like the Royal National Lifeboat Institution (RNLI) and HM Coastguard run education campaigns about the dangers of the ocean. The Canal & River Trust, the UK’s largest canal charity, recently developed a school education pack for teenagers highlighting water safety.
Parents can also shape how teens interact with water. In Nottingham, the charity called Open Water Education Network was founded in memory of Owen Jenkins, a 12-year-old boy who drowned while trying to save two girls in difficulty. As well as teaching young people about the dangers of open water and the importance of self rescue, this charity empowers parents to talk to teens even if they seem to ignore parental advice.
Talking to teenagers about safety isn’t easy. Here’s how to do it in a way that’s honest, effective and grounded in care.
1. Talk just before they go
Rules work best when they’re short, consistent and repeated. Before a trip to the beach or river, take five minutes to remind your teen of your family’s water safety rules. Repetition builds habits. Remind them not to swim after dark or alone and explain what to do if someone’s in trouble (call for help, don’t jump in).
2. Share real-life stories
Stories help bring home the reality of water risk, especially for teens who can feel invincible in an all-male group without any supervision. While on a lads holiday on the Northumberland coast, 16-year-old called Evan saved himself from drowning in a rip tide by laying on his back to stay afloat. Eventually, a surfer managed to paddle out and reach him, and an rescue lifeboat also came to the scene. Evan recovered after treatment in hospital for hypothermia.
Another heartbreaking story of Liam Hall, a teenager who drowned while out in a dinghy with friends in Sunderland, demonstrates how quickly things can escalate in the sea.
Not all stories end in tragedy. A group of teenagers from East Sussex made the life-saving decision to stay out of the water, using a life ring to help two swimmers in trouble, proving that staying on shore can save lives.

oneinchpunch/Shutterstock
3. Discuss group dynamics
Female teens can play a powerful role in promoting water safety, especially in mixed-gender peer groups where social dynamics can significantly influence behaviour. Research shows that all-boy groups are more likely to engage in risk-taking activities. When girls are present, especially those who feel confident speaking up, risky behaviour often decreases.
Parents can empower girls to speak up if someone suggests swimming in dangerous conditions or places and promote safety strategies like the RNLI’s “call, tell and throw” approach. By reinforcing these behaviours, teen girls can become leaders in lifesaving culture, not only keeping themselves safe but influencing their peers to make smarter choices too.
4. Deflate false sense of confidence
Stick to the facts and be honest about the dangers. Drowning can happen within seconds, even when someone is a strong swimmer. Most drownings occur in open water, not swimming pools. Teenagers need to understand how the effects of cold water shock, fast currents and submerged objects can quickly turn a fun day into a fatal one.
5. Make brave choices
Teens don’t drown because they’re bad swimmers. They drown because they made a poor decision in a high-risk moment. Teaching safety early (before they start taking unsupervised risks) helps shape smarter thinking later.
Parents can model care, calmness and emotional awareness. Show them that bravery isn’t about bravado. It’s about looking out for your mates and making good choices. Fathers can play a powerful role in framing what strength looks like. Research shows that fathers who show empathy and emotional intelligence teach children how to be resilient during high-pressure moments. Emphasise that calm decision-making when in danger or choosing not to jump into the water under peer pressure doesn’t make a boy weak. It makes him wise. Talk to your sons about how real masculinity means thinking clearly, not reacting emotionally.
Teenagers can feel invincible. Be honest. Tell them you love them and that you trust them to make good decisions. Talking about safety is one of the most powerful things a family can do. Water safety begins at home with all of us.
Swimming, sailing, even just building a sandcastle – the ocean benefits our physical and mental wellbeing. Curious about how a strong coastal connection helps drive marine conservation, scientists are diving in to investigate the power of blue health.
This article is part of a series, Vitamin Sea, exploring how the ocean can be enhanced by our interaction with it.![]()
Jill Nash, Senior Lecturer in Advertising and Marketing Communications, Bournemouth University
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Hidden hunger amongst older adults: A short film co-produced with local communities
Credit to Dr Kate Jupp, PIER officer, Stevie Corbin-Clarke and Misha Naran (research assistants) for their amazing work on the Hidden Hunger project with BCP Access to Food Partnership. Links to the research report and associated film can be found in BCP Access to Food Partnership’s press release below.
Isolation and loneliness are big issues facing older adults in our communities. This project hears from people in the BCP area and their struggles and difficulties around food, connection and the stigma attached to asking for support. BCP’s Access to Food Partnership wanted to better understand local observations that ‘older adults are less likely to use foodbanks and have a higher level of attendance at lunch club settings’.
In collaboration with Christchurch Community Partnership and Bournemouth University’s PIER (Public Involvement in Education and Research) team, the project worked closely with Highcliffe Lunch Club to better understand the barriers as well as the solutions that will enable better access to relevant support for older people experiencing food insecurity.
The BU PIER Community Researcher Model enables participants with lived experience of the topic being explored to feel comfortable in sharing their insight as well as building confidence in becoming peer mentors and community researchers.
The conversations by community researchers identified the following themes:
- Basic Needs Not Being Met
- Broadening from Poverty
- Benefits of Lunch Clubs
- Asking For Help
- Someone to Trust
“A significant learning for us from his project was that access to food for some older people was not only about poverty. It was often about mobility, frailty and tied up with not wanting to ask for help or be seen as a burden. Having someone they could just ask, was the key to making a difference”. BU Research team
The research has prompted partners to take action and develop their practice using the lessons learnt, including:
- Christchurch Community Partnership (CCP) has established a new monthly Sunday Roast project They have also started a completely new weekly CCP Lunch Club which includes more time either side of a meal for people to socialise (mobility issues meant eating and talking is harder for some attendees) as well as supporting the Greystones lunch club. “We were surprised at how much food insecurity there is for many of our older residents and the Hidden Hunger research has both informed and shaped our provision going forward”. Rev. Sandra Prudom CEO of CCP.
- Partners will use the learnt ‘community researcher model’ to reach other seldom heard voices within our community.
- Bournemouth University will help build capacity for community groups, staff and volunteers to have the skills and confidence to lead and conduct their own research
The Access to Food Partnership is a group of over 70 community food organisations and many others from the public and voluntary sector. They will continue to connect and deliver better outcomes for those experiencing food insecurity. Alistair Doxat-Purser (Chair of Access to Food and CEO of Faithworks) said:
“The findings from this research project are very timely as demand at foodbanks from those over 65 starts to grow. Relational support as well as practical help is becoming more and more what the Access to Food Partnership stands for – and magnificently demonstrates day in, day out.”
The partners have recently produced a film to go alongside the report to share the findings of the project, which can be viewed in the ‘Research section’ here:
Resources for Access to Food Partnership members | BCP and Hidden Hunger Film
Thanks
Michael
Michael French
Community Food Co-ordinator
Public Health and Communities Directorate
AI learning to read emotions from motion….
 This is Dr Roya Haratian participating in data collection to help develop AI which can read emotions from motion!
This is Dr Roya Haratian participating in data collection to help develop AI which can read emotions from motion!
This is our Higher Education Innovation Fund (HEIF) project HORSENSE VR. We are developing a game which enables participants to play with horses in a virtual environment to develop calmness and reduce anxiety.
We are working with external partners – our team is Dr Roya Haratian, Prof Fred Charles, Prof Ann Hemingway, Dr Xun He, Harriet Laurie MBE (The HorseCourse) Liucheng Guo (Tg0), Paul Brown.
Bournemouth University eHealth paper cited 40 times!
Yesterday, ResearchGate alerted us that the paper ‘Midwives’ views towards women using mHealth and eHealth to self-monitor their pregnancy: A systematic review of the literature’ [1] had reached 40 citations! This paper has four Bournemouth University (BU) authors and one author, Prof. Gary Smith, who was FHSS Visiting Professor at the time of publication. This literature review, published in 2020, sought midwives’ perspectives on women self-monitoring their pregnancy using eHealth and mHealth.
 The paper fund that midwives generally held ambivalent views towards the use of eHealth and mHealth technologies in antenatal care. They acknowledged the potential benefits of such technologies, such as their ability to modernise antenatal care and to help women make more informed decisions about their pregnancy. However, midwives were quick to point out the risks and limitations of these, such as the accuracy of conveyed information, and negative impacts on the patient-professional relationship.
The paper fund that midwives generally held ambivalent views towards the use of eHealth and mHealth technologies in antenatal care. They acknowledged the potential benefits of such technologies, such as their ability to modernise antenatal care and to help women make more informed decisions about their pregnancy. However, midwives were quick to point out the risks and limitations of these, such as the accuracy of conveyed information, and negative impacts on the patient-professional relationship.
 This paper will contribute to our recently awarded NIHR funding to tackle inequalities in UK maternal healthcare as part of the NIHR Challenge Call: Maternity Disparities Consortium. Profs Vanora Hundley and Edwin van Teijlingen from the Centre for Midwifery and Women’s Health, and Prof. Huseyin Dogan and Dr. Deniz Cetinkaya from the Department of Computing and Informatics collaborate in MIHERC (Maternal & Infant Health Equity Research Centre). MIHERC is led by Prof. Hora Soltani at Sheffield Hallam University, and it is a partnership with Bournemouth University, the City of Doncaster Council and South Yorkshire Digital Health Hub as well as several charities and voluntary organisations. Prof. Dogan has recently been appointed the co-lead for the “Digital, data, monitoring, evaluation and implementation science” work stream of the NIHR Maternity Disparities consortium.
This paper will contribute to our recently awarded NIHR funding to tackle inequalities in UK maternal healthcare as part of the NIHR Challenge Call: Maternity Disparities Consortium. Profs Vanora Hundley and Edwin van Teijlingen from the Centre for Midwifery and Women’s Health, and Prof. Huseyin Dogan and Dr. Deniz Cetinkaya from the Department of Computing and Informatics collaborate in MIHERC (Maternal & Infant Health Equity Research Centre). MIHERC is led by Prof. Hora Soltani at Sheffield Hallam University, and it is a partnership with Bournemouth University, the City of Doncaster Council and South Yorkshire Digital Health Hub as well as several charities and voluntary organisations. Prof. Dogan has recently been appointed the co-lead for the “Digital, data, monitoring, evaluation and implementation science” work stream of the NIHR Maternity Disparities consortium.

Reference:
- Vickery, M., van Teijlingen, E., Hundley, V., Smith, G. B., Way, S., Westwood, G. (2020). Midwives’ views towards women using mHealth and eHealth to self-monitor their pregnancy: A systematic review of the literature. European Journal of Midwifery, 4(Sept.), 1-11. https://doi.org/10.18332/ejm/126625
Conversation article: Wishcycling – how ‘eco-friendly’ labels confuse shoppers and make recycling less effective
Anastasia Vayona writes for The Conversation about new research showing how misleading environmental labelling and claims are confusing shoppers and making recycling more confusing…
Wishcycling: how ‘eco-friendly’ labels confuse shoppers and make recycling less effective

Anastasia Vayona, Bournemouth University
Have you ever thrown something in the recycling bin, hoping it’s recyclable? Maybe a toothpaste tube, bubble wrap or plastic toy labelled “eco-friendly”?
This common practice, known as “wishcycling”, might seem harmless. But my colleagues and I have published research that shows misleading environmental claims by companies are making recycling more confusing – and less effective.
This kind of marketing leads to greenwashed consumer behaviour — when people believe they are making environmentally friendly choices, but are being misled by exaggerated or false claims about how sustainable a product is.
We surveyed 537 consumers from 102 towns across the UK to explore a simple question: is there a link between greenwashed consumer behaviour and wishcycling? We wanted to find out whether they feed into each other, what drives them both, and how consumers perceive the connection.
What makes this issue particularly interesting is its psychological foundation. We argue that modern consumers have been burdened with a responsibility that may be beyond their capacity: deciding what to do with product packaging after use.
Many people are unprepared, undereducated or simply unaware of the full effect of their choices — and why should they be? This is a burden that should not rest on their shoulders. Into this gap has stepped recycling, presented as the solution. Consumers are led to believe that by recycling, they are doing their part to help the environment.
However, when products carry environmental claims or symbols — even vague ones like a green leaf, green banner or “earth-friendly” label — consumers often fall prey to what we call the “environmental halo effect”. This cognitive bias causes people to attribute positive environmental qualities to the entire product, including how it’s disposed of, even when those claims may not be accurate.
Surprisingly, our study reveals that environmentally conscious consumers can be most susceptible to this effect. Their strong environmental values may make them more inclined to trust green marketing claims, even when those claims are vague or misleading.

Billion Photos/Shutterstock
Driven by their desire to make sustainable choices, these consumers often accept green marketing claims at face value, assuming that environmental claims reflect genuine efforts toward sustainability.
Even more intriguingly, we found that people with higher levels of education tend to trust companies’ environmental claims more readily, especially when these companies present themselves as environmentally responsible.
This all leads to more wishcycling, not less. When companies talk about their environmental ethos and social responsibility, we’re more likely to believe their packaging is recyclable – even when it isn’t.
Our research also suggests that younger consumers, despite being generally more environmentally aware, are more likely to wishcycle. While millennials and generation Z often express strong environmental values, they’re also often more likely to contaminate recycling streams by throwing in non-recyclable items.
The future is circular
The solution is not to stop caring for the environment, but to channel that care more effectively. At the heart of this approach is the concept of a circular economy, where products and materials are reused, refurbished and recycled, rather than discarded.
The answer isn’t just better recycling – it’s better packaging design and corporate responsibility from the start. While we as consumers should continue doing our part, the primary burden should rest with manufacturers to create packaging that’s genuinely recyclable or reusable, not just marketed as “eco-friendly”.
This means implementing clear, standardised labelling that leaves no room for confusion, using packaging made from single, easily recyclable materials, and designing for reuse and refill systems.
On February 11 2025, the EU enacted a new packaging and packaging waste directive. This is designed to reduce packaging waste and support a circular economy by setting rules for how packaging should be made, used and disposed of throughout its lifecycle.
Until these systemic changes are fully implemented, we need to be both environmentally conscious and critically aware consumers. But it’s important to remember: while our daily choices and actions matter, the key to real change lies in pushing for corporate and policy-level transformation of our packaging systems.
By designing out waste, the circular economy offers a sustainable model that can guide these changes and reduce our dependence on single-use packaging. Hopefully, this can inspire us to improve current practices and keep finding better ways to do things, leading to a more sustainable and resilient future.
Don’t have time to read about climate change as much as you’d like?
Get a weekly roundup in your inbox instead. Every Wednesday, The Conversation’s environment editor writes Imagine, a short email that goes a little deeper into just one climate issue. Join the 45,000+ readers who’ve subscribed so far.![]()
Anastasia Vayona, Postdoctoral Research Fellow in Social Science and Policy, Faculty of Science and Technology, Bournemouth University
This article is republished from The Conversation under a Creative Commons license. Read the original article.
Capturing the Power of Heat: NCEM’s Breakthrough in Clean Energy Storage Gains Global Recognition
The NanoCorr, Energy & Modelling (NCEM) Research Group, led by Professor Zulfiqar Khan at Bournemouth University, has made pioneering developments in the field of thermal energy storage, an area critical to the future of renewable energy. Their groundbreaking work in enhancing the performance of latent heat storage systems using phase change materials (PCMs) has been featured on the International Atomic Energy Agency (IAEA) website—marking a prestigious global endorsement of their innovations in clean energy technologies.

A Leap Toward Sustainable Energy
As the world shifts from fossil fuels to renewable sources, the ability to capture and store energy efficiently is a central challenge. PCMs—materials that absorb and release heat during phase transitions (like melting and solidifying)—offer an ingenious solution. NCEM’s research focuses on improving these materials’ thermal conductivity, stability, and compatibility with various containers, making energy storage more efficient, stable, and commercially viable.
Their study reviews and categorises organic paraffins and inorganic salt hydrates, the most promising groups of PCMs, highlighting enhancements like encapsulation, multi-PCM integration, and advanced container geometries. These techniques significantly boost energy capture rates and storage capacity, making clean energy more practical for widespread use.
Real-World Impact and Innovation
Backed by five industry-funded and match-funded projects, NCEM’s efforts have not only led to commercial patents in the UK and USA, but have also influenced engineering solutions for solar heating, industrial heat recovery, and smart building technologies. These contributions align strongly with several United Nations Sustainable Development Goals (UNSDGs):
Goal 7: Affordable and Clean Energy
Goal 9: Industry, Innovation, and Infrastructure
Goal 12: Responsible Consumption and Production
Goal 17: Partnerships for the Goals
Global Recognition: Why the IAEA Feature Matters
The International Atomic Energy Agency (IAEA), an influential global body under the United Nations, works to promote the peaceful use of nuclear and clean energy technologies. Being featured on their platform is a significant milestone—it underscores the global relevance, technical merit, and strategic value of Professor Khan’s research. It also places Bournemouth University and NCEM at the heart of international discussions on sustainable energy systems.
This acknowledgment by the IAEA is a testament to the NCEM team’s commitment to tackling real-world problems with innovative science. It further demonstrates the potential of UK-led clean energy solutions to contribute to a low-carbon, energy-secure future for all.
A Bright Future for Clean Energy
The research led by Professor Zulfiqar Khan exemplifies how innovative materials science and engineering can drive change on a global scale. With continued support and collaboration between academia and industry, NCEM is set to play a pivotal role in accelerating the transition to clean, resilient energy systems worldwide.
Acknowledgements: Dr Zakir Khan (NCEM ex PGR/ Post Doc) and Professor A Ghafoor.
BU research on road network efficiency reported in Times of India
BU research , led by PhD graduate Dr. Assemgul Kozhabek and Dr. Wei Koong Chai, on characterising efficiency of road networks in big populous cities around the world was covered by the Times of India, the world’s largest selling English-language daily in the world. The article reported the findings from BU’s work, specifically highlighted its insights into the structure, properties and efficiency of road networks in relevant cities in India.
Article: “Study rates ‘efficiency’ of city road network” March 17 2025, https://timesofindia.indiatimes.com/city/ahmedabad/study-rates-efficiency-of-city-road-network/articleshow/119086477.cms.
BU collaborates with University of Exeter on modelling innovation adoption
Bournemouth University (BU) has collaborated with the University of Exeter on modelling innovation adoption diffusion. The work, led by Dr. Wei Koong Chai in BU, draw on the epidemic theory and model the diffusion dynamics considering (1) the role of network structures in dictating the spread of adoption and (2) how individual’s characteristic/capability influences the path of diffusion (e.g. an individual may have different attitude or ability towards adopting a new innovation). A positive adoption decision is related to the number of neighbors adopting the innovation. The neighbors decisions are, in turn, dependent on their own neighbors and so, it forms a complex cascading inter-dependent relationship between the different individuals in the network. As such, each node in the network is unique and its relevant adoption rate must be considered separately conditioned with the activities occurring in the network over time.
The model offers insights into how the network spectrum affects the innovation exposure rate and spreading of innovation individually and across communities with different adoption behaviours. It also illustrates the effects of the embedded social structure and the characteristics of individuals in the network on the path of innovation diffusion via two use cases: (i) innovation adoption of EU countries in a Single Market Programme and (ii) innovation adoption of specific class of technology (specifically financial technologies (FinTech)).
Reference:
Duanmu, JL., Chai, W.K. Modelling innovation adoption spreading in complex networks. Appl Netw Sci 10, 10 (2025). https://doi.org/10.1007/s41109-025-00698-8
An Audience with… Bob Wilson
Last Monday, the BU Business School and the Sport & Physical Activity Research Centre (SPARC) were delighted to welcome Mr Bob Wilson, OBE, for a special session.
Launching the annual ‘SPARC Audience with ….’ Series, this inaugural event explored the multiple careers of Bob Wilson: a former Arsenal and Scotland goalkeeper, a presenter and broadcaster, the first ever specialist goalkeeper coach, and founder of the national charity, The Willow Foundation.

Professor Mike Silk in conversation with Bob Wilson OBE
During An Audience with Bob Wilson, Bob touched on a multitude of topics that are germane to the BU community, covering topics that included the sport media, the life of a professional athlete, (national) identity politics in sport, the development of the women’s game, football as ‘work’, injury, the commercial spectacle of the modern game, and some of the broadcast personalities with whom he has worked.
Bob regaled the audience with stories from his early playing days explaining how his Father refused to let him join Manchester United, insisting instead he completed teacher training at Loughborough College so he could hold down a ‘proper job’. We celebrated some of Bob’s accomplishments, including being an ever present in the 1970/71 double winning season, with Bob sharing his winning medals from both the League and FA Cup final and his international caps. He spoke about the ways in which he was treated by the Scottish press when—as an Englishman—he was picked to represent the Scottish national team. He spoke of losing his older brothers in the second World War and how his family reacted to Bob idolising the playing style of ex-German soldier and Manchester City Goalkeeper, Bert Trautman.
In addition to appreciating his football career, we spoke about how he transitioned to a career in the media—going on to present programmes such as Grandstand, Match of the Day, BBC Breakfast and Sportsnight on the BBC as well as League Cup, FA Cup, UEFA Champions League and World Cup coverage on ITV. Bob gave fascinating insights into the creation of his own programme, Football Focus, as well as his unique ‘running’ reporting style from coverage of the London Marathon. We heard insights into the tensions and dynamics of live television when Bob told us how he and the production team dealt with being live on air on the Saturday afternoon of the 15th April, 1989 as he fronted Grandstand and bought the country to a standstill with coverage of the unfolding Hillsbrorough disaster.
Bob provided a unique lens into the development of the game of football. He was the first ever goalkeeper coach, staying on at Arsenal after his playing days—juggling coaching with his media career—providing coaching for the likes of Pat Jennings, John Lukic and David Seaman. This was a role he did for free until the arrival of Arsene Wenger as Arsenal Manager who insisted he be paid for his duties. Bob was, in many ways, the pioneer for the plethora of specialist coaches that exist today in the modern game.
Finally, we spoke of the incredible achievements of the Willow foundation, the charity he and his wife, Megs, set up in 1999 in memory of his daughter Anna who died from cancer at 31 years of age. The Willow Foundation—so named after his own football nickname, Willow—provides psychological and emotional support for seriously ill 16-40 year olds through the provision of special day experiences. Bob explained how, to date, the Willow Foundation has in 25 years supported over 22,000 families.
With some great questions from the audience, hilarious stories and insightful discussion, An Audience with Bob Wilson saw staff and students from across the institution engage with a sporting and broadcasting legend.
Speaker @ NLP Healthcare Summit 2025: Evaluating LLMs in Understanding Image Series Textual Narratives

📢 This is a short and final notice that already today 02 April 2025 I am going to showcase the advances made a part of Marking Medical Images with NLP at Bournemouth University and National Centre for Computer Animation, Bournemouth University at NLP Healthcare Summit 2025.
🗓️ Save an event in Google Calendar: https://lnkd.in/eHfMyRUk
⏲️ Time: 8.10 PM (Europe / London Timezone)
🙌 Feel free to join and ask me any related questions
> What is going to be about?
> We overview the problem of extracting image series acquisition aspects from short text medical reports in the domain of HCC liver cancer imaging. Our focus is to evaluate performance of various stock LLM model in out-of-the-box condition using instruction based approach. The goal is to answer the question: to what extent we can trust LLM for retrieving medical aspects and depending of the scale of LLM.
Dr. Nicolay Rusnachenko
Research Fellow at Centre For Applied Creative Technologies PLUS (CFACT+)
Bournemouth University
Nepal Family Cohort Study dissemination event
 Colleagues working on our Nepal Family Cohort Study (NeFCoS) presented baseline data at a dissemination programme held today (March 28th) in Everest Hotel, Kathmandu. Bournemouth University is a key partner in this unique long-term follow-up study in Nepal. Our large international team is led by Dr. Om Kurmi, Associate Professor Research in the Centre for Healthcare and Communities at Coventry University. The Bournemouth University (BU) team comprises Dr. Pramod Regmi (Principal Academic-International Health), Dr. Edwin van Teijlingen (Professor of Reproductive Health), Dr. Rebecca Neal (Principal Lecturer in Exercise Physiology) and Dr. Vanora Hundley (Professor of Midwifery).
Colleagues working on our Nepal Family Cohort Study (NeFCoS) presented baseline data at a dissemination programme held today (March 28th) in Everest Hotel, Kathmandu. Bournemouth University is a key partner in this unique long-term follow-up study in Nepal. Our large international team is led by Dr. Om Kurmi, Associate Professor Research in the Centre for Healthcare and Communities at Coventry University. The Bournemouth University (BU) team comprises Dr. Pramod Regmi (Principal Academic-International Health), Dr. Edwin van Teijlingen (Professor of Reproductive Health), Dr. Rebecca Neal (Principal Lecturer in Exercise Physiology) and Dr. Vanora Hundley (Professor of Midwifery).

- Kurmi, O.P., Chaudhary, N., Delanerolle, G., Bolton, C.E., Pant, P.R., Regmi, P., Gautam, S., Satia, I., Simkhada, P., Kyrou, I., Sigdel, T.K., Hundley, V., Dali, P.R., Lokke, A., Hubert Lam, K.B., Bennett, D., Custovic, A., van Teijlingen, E., Gill, P. Randeva, H., O’Byrne, P.M., and Nepal Family Cohort Collaborators Group, Nepal Family Cohort Study: A Study Protocol, BMJ Open 14:e088896. doi:10.1136/ bmjopen-2024-088896
Presenting Advances in Marking Medical Images at NLP Healthcare Summit 2025

📢 The NLP Heathcare Summit 2025 is nearly in a week. This year, I am looking forward to showcase advances that were made at Bournemouth University in Understanding Medical Concepts via LLM in Textual Series Narratives of Imaging Series ⚕️
In this talk we cover LLM evaluation concepts through the detailed overview of manual reporting directions in liver cancer imaging. We cover radiological imaging aspects that doctors tend to mention ✍️ and see how these aspects could be retrieved by LLM 🤖 by means of NLP and IR techniques. We provide recommendations on model scale choice and findings out of result analysis.
These advances were achieved while at Centre for Applied Creative Technologies CfACTs+ by working on “Marking Medical Image Reports Automatically with Natural Language Processing (NLP-MMI)” project.
Dr. Nicolay Rusnachenko
Research Fellow at Centre For Applied Creative Technologies PLUS (CFACT+)
Bournemouth University
Reading about Positionality
This week ResearchGate informed us that the paper ‘The Importance of Positionality for Qualitative Researchers‘ by Bournemouth University M.Res. student Ms. Hannah Gurr has been read over 800 times. The co-authors are Hannah’s supervisors Dr. Louise Oliver, Dr. Orlanda Harvey and Prof. Edwin van Teijlingen in the Faculty of Health & Social Sciences (FHSS), and one of Nepal’s foremost sociology of health and illness researchers Prof. Madhusudan Subedi. 
The paper is of particular interest for qualitative and mixed-methods researchers as these researchers are especially required to be critically reflective and explain to readers their positionality on their work. This account can be relatively straightforward, but there are occasions when this process of reflection and outlining one’s positionality is much more complicated. This method-paper explains this process. It outlines, using examples of different occasions and situations, where and why such complications may arise, for example, around values and personal experiences. It concludes with further practical advice on writing the section on positionality for novice social scientists. The journal in which this methodological paper is published is Open Access and therefor freely available to read for anybody across the globe.
Reference:
- Gurr, H., Oliver, L., Harvey, O., Subedi, M., van Teijlingen, E. (2024) The Importance of Positionality for Qualitative Researchers, Dhaulagiri Journal of Sociology and Anthropology 18(1): 48-54,
New Social Work Education paper published
-
Frampton, M., Schiller, U., Parker, J., Hartogh, T., Arlinghaus, G.A., Fry, A.D.J., Autio, K., Ndhlovu, N. (2025): Using the arts in social work education for short-term European mobility: evaluating student experiences on an Erasmus+ blended intensive program, Social Work Education, DOI: 10.1080/02615479.2025.2466707
NCCA Research: Ensuring the Quality of Autonomous Intelligent Systems through the Guide to Ethical Assessment of the Product
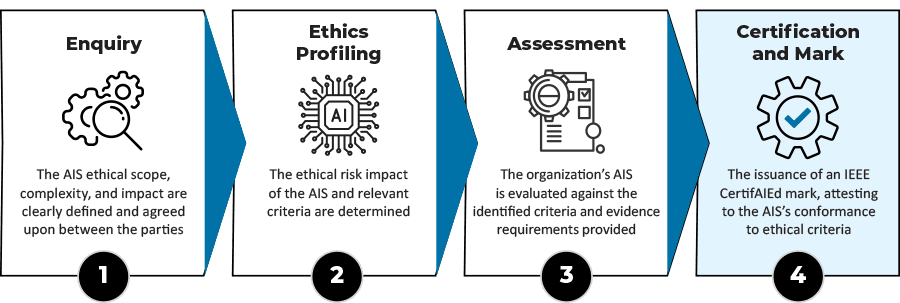
So far, I successfully complete IEEE Standards Association | IEEE SA training program 🎓, dedicated to guide Ethical Assessment and Product Improvement using the CertifAIEd framework.
The following Wednessday 26th of March 2025, as a part of NCCA Research Seminars, I am looking forward to briefly demostrate the potentials of earned skills in the following talk:
Title: Ensuring the Quality of Autonomous Intelligent Systems: A Guide to Ethical Assessment and Product Improvement using the IEEE CertifAIEd framework.
Description: Many products employ Autonomous Intelligent Systems (AIS) including: transportation (self-driving cars), manufacturing, retail / customer service, healthcare, finance, education (AI learning platforms). The use of a quality framework to manage the risks of AIS is crucial to protect users and grow product adoption. The standards association IEEE offers the ability to evaluate the quality of any AIS using an evaluation framework – CertifAIEd. Having recently completed the first stage of IEEE CertifAIEd training, I will present an overview of the potentials of CertifAIEd and the route to become an authorized assessor.
Dr. Nicolay Rusnachenko
Research Fellow at Centre For Applied Creative Technologies PLUS (CFACT+)
Bournemouth University
Presenting BU’s kidney disease research in Manchester
 Tuesday 18th March Drs. Pramod Regmi and Nirmal Aryal from the Department of Nursing Sciences presented our work on risk of kidney disease in Nepalese migrant workers in the Middle East and Malaysia. The presentation was at the Project Impact Seminar in the Whitworth Gallery in Manchester organised by The Colt Foundation, the funder of our research.
Tuesday 18th March Drs. Pramod Regmi and Nirmal Aryal from the Department of Nursing Sciences presented our work on risk of kidney disease in Nepalese migrant workers in the Middle East and Malaysia. The presentation was at the Project Impact Seminar in the Whitworth Gallery in Manchester organised by The Colt Foundation, the funder of our research. 
The title was: ‘Risk of kidney injury among returnee Nepalese migrants: a mixed-methods’ which was conducted in the one of areas of Nepal with a high proportion of people working abroad as migrant labourers. Over the past decade there have been increasing concerns about possible chronic kidney disease (CKD) in migrant workers returning to Nepal. 
This important study has resulted in one publication in PLoS One (1) and one more recently submitted. The event was good for networking to discuss possible collaborations. We also had the pleasure of meeting former colleagues from the University of Aberdeen, now based at the University of Stirling. This dissemination event is the latest in a long line of research publications focusing on the health and well-being of migrant workers from Nepal from the BU team in the Faculty of Health & Social Sciences [1-35].
Prof. Edwin van Teijlingen
Centre for Midwifery & Women’s Health
References:
- Regmi, P., Aryal, N., Bhattarai, S., Sedhain, A., KC, R.K. and van Teijlingen, E. (2024) Exploring lifestyles, work environment and health care experience of Nepalese returnee labour migrants diagnosed with kidney-related problems, PLoS One 19(8): e0309203. https://doi.org/10.1371/journal.pone.0309203

- Paudyal, P., Wasti, S.P., Neupane, P., Sapkota, J.L., Watts, C., Kulasabanathan, K., Silwal, R., Memon, A., Shukla, P., Pathak, R.S., Michelson, D., Beery, C., Moult, A., Simkhada, P., van Teijlingen, E., Cassell, J. 10, (2025) Coproducing a culturally sensitive storytelling video intervention to improve psychosocial well-being: a multimethods participatory study with Nepalese migrant workers, BMJ Open 15:e086280. doi: 10.1136/bmjopen-2024-086280
- Mahato, P., Bhusal, S., Regmi, P., van Teijlingen, E. (2024). Health and Wellbeing Among Nepali Migrants: A Scoping Review. Journal of Health Promotion, 12(1): 79–90. https://doi.org/10.3126/jhp.v12i1.72699
- Paudyal, A.R., Harvey, O., van Teijlingen, E., Regmi, P. R., Sharma, C. (2024). Returning Home to Nepal after Modern Slavery: Opportunities for Health Promotion. Journal of Health Promotion, 12(1): 125–132. https://doi.org/10.3126/jhp.v12i1.72713
- Simkhada, P.P., van Teijlingen, E., Gurung, M., Bhujel, S., Wasti, S.P. (2024) Workplace harassment faced by female Nepalese migrants working aboard, Global Health Journal 8(3): 128-132. https://www.sciencedirect.com/science/article/pii/S241464472400040X
- Regmi, P., Aryal, N., van Teijlingen, E., KC, R.K., Gautam, M. and Maharjan, S. (2024). A Qualitative Insight into Pre-Departure Orientation Training for Aspiring Nepalese Migrant Workers. Tropical Medicine and Infectious Disease, 9 (7).
- Chaudhary, M.N., Lim, V.C., Faller, E.M., Regmi, P., Aryal, N., Zain, S.N.M., Azman, A.S. and Sahimin, N. (2024). Assessing the basic knowledge and awareness of dengue fever prevention among migrant workers in Klang Valley, Malaysia. PLoS ONE, 19 (2).
- Aryal, N., Regmi, P., Adhikari Dhakal, S., Sharma, S. and van Teijlingen, E. (2024). Moral panic, fear, stigma, and discrimination against returnee migrants and Muslim populations in Nepal: analyses of COVID-19 media content. Journal of Media Studies, 38 (2), 71-98.
- Chaudhary, M.N., Lim, V.C., Sahimin, N., Faller, E.M., Regmi, P., Aryal, N. and Azman, A.S. (2023). Assessing the knowledge of, attitudes towards, and practices in, food safety among migrant workers in Klang Valley, Malaysia. Travel Medicine and Infectious Disease, 54.
- Adhikari, Y., Regmi, P., Devkota, B. and van Teijlingen, E. (2023). Forgotten health and social care needs of left-behind families of Nepali migrant workers. Journal of Health Promotion, 10, 1-4.
- Regmi, P., Simkhada, P., Aryal, N., van Teijlingen, E. (2022) Excessive mortalities among migrant workers: the case of the 2022 FIFA World Cup. Europasian Journal of Medical Sciences, 4:31-32. https://doi.org/10.46405/ejms.v4i0.455
- Regmi, P., Dhakal Adhikari, S., Aryal, N., Wasti, S.P., van Teijlingen, E. (2022) Fear, Stigma and Othering: The Impact of COVID-19 Rumours on Returnee Migrants and Muslim Populations of Nepal, International Journal of Environmental Research & Public Health 19(15), 8986; https://doi.org/10.3390/ijerph19158986
- Simkhada, P., van Teijlingen, E. and Regmi, P. (2022). Migrant Workers in Qatar: Not just an important topic during the FIFA World Cup 2022. Health Prospect: Journal of Public Health, 21 (3), 1-2.
- Simkhada, B., Sah, R.K., Mercel-Sanca, A., van Teijlingen, E., Bhurtyal, Y.M. and Regmi, P. (2021). Perceptions and Experiences of Health and Social Care Utilisation of the UK-Nepali Population. Journal of Immigrant and Minority Health, 23 (2), 298-307.
- Aryal, N., Sedhain, A., Regmi, P.R., KC, R. K., van Teijlingen, E. (2021). Risk of kidney health among returnee Nepali migrant workers: A survey of nephrologists. Asian Journal of Medical Sciences, 12(12), 126–132. https://doi.org/10.3126/ajms.v12i12.39027
- Aryal, N., Regmi, P.R., Sedhain, A., KC, R.K., Martinez Faller, E., Rijal, A., van Teijlingen, E. (2021) Kidney health risk of migrant workers: An issue we can no longer overlook. Health Prospect 20(1):15-7
- Aryal, N., Regmi, P.R., van Teijlingen, E., Trenoweth, S., Adhikary, P. and Simkhada, P., (2020). The impact of spousal migration on the mental health of Nepali women: A cross-sectional study. International Journal of Environmental Research and Public Health, 17 (4).
- Regmi, P., Aryal, N., van Teijlingen, E., Adhikary, P. (2020) Nepali migrant workers and the need for pre-departure training on mental health: a qualitative study, Journal of Immigrant & Minority Health https://link.springer.com/content/pdf/10.1007/s10903-019-00960-z.pdf
- Adhikary, P., Aryal, N., Dhungana, R.R., KC, R.K., Regmi, P., Wickramage, K.P., Duigan, P., Inkochasan, M., Sharma, G.N., Devkota, B., van Teijlingen, E. and Simkhada, P. (2020). Accessing health services in India: Experiences of seasonal migrants returning to Nepal. BMC Health Services Research, 20 (1), 992.
- Regmi, P., van Teijlingen, E., Mahato, P., Aryal, N., Jadhav, N., Simkhada, P., Zahiruddin, Q.S., Gaidhane, A. (2019) The Health of Nepali Migrants in India: A Qualitative Study of Lifestyles and Risks. International Journal of Environmental Research and Public Health, 16 (19). https://doi.org/10.3390/ijerph16193655
- Adhikary P, van Teijlingen E. (2019) Support networks in the Middle East & Malaysia: A qualitative study of Nepali returnee migrants’ experiences’ – International Journal of Occupational Safety and Health 9(2): 31-35.
- Aryal, N., Regmi, P.R., Faller, E.M., van Teijlingen, E., Khoon, C.C., Pereira, A., Simkhada, P. (2019) Sudden cardiac death and kidney health related problems among Nepali migrant workers in Malaysia. Nepal Journal of Epidemiology, 9 (3), 788-791. https://doi.org/10.3126/nje.v9i3.25805
- Adhikary P, van Teijlingen E., Keen S. (2019) Workplace accidents among Nepali male workers in the Middle East and Malaysia: A qualitative study, Journal of Immigrant & Minority Health 21(5): 1115–1122. https://link.springer.com/article/10.1007/s10903-018-0801-y
- Dhungana, R.R., Aryal, N., Adhikary, P., Kc, R.K., Regmi, P.R., Devkota, B., Sharma, G.N., Wickramage, K., Van Teijlingen, E. and Simkhada, P. (2019). Psychological morbidity in Nepali cross-border migrants in India: A community based cross-sectional study. BMC Public Health, 19 (1).
- Aryal, N., Regmi, P.R., van Teijlingen, E., Simkhada, P. and Mahat, P. (2019). Adolescents left behind by migrant workers: a call for community-based mental health interventions in Nepal. WHO South-East Asia journal of public health, 8 (1), 38-41.
- Simkhada, P.P., van Teijlingen, E.R., Gurung, M., Wasti, S. (2018) A survey of health problems of Nepalese female migrants workers in the Middle-East & Malaysia, BMC International Health & Human Rights 18(4): 1-7. http://rdcu.be/E3Ro.
- Simkhada, P., van Teijlingen, E., Sharma, A., Bissell, P., Poobalan, A., Wasti, S.P. (2018) Health consequences of sex trafficking: A systematic review, Journal of Manmohan Memorial Institute of Health Sciences, 4(1): 130-49.
- Adhikary P, Sheppard, Z., Keen S., van Teijlingen E. (2018) Health and well-being of Nepalese migrant workers abroad, International Journal of Migration, Health & Social Care 14(1): 96-105. https://doi.org/10.1108/IJMHSC-12-2015-0052
- Adhikary, P, Sheppard, Z., Keen, S., van Teijlingen, E. (2017) Risky work: accidents among Nepalese migrant workers in Malaysia, Qatar & Saudi Arabia, Health Prospect 16(2): 3-10.
- Simkhada, P.P., Regmi, P.R., van Teijlingen, E., Aryal, N. (2017) Identifying the gaps in Nepalese migrant workers’ health and well-being: A review of the literature. Journal of Travel Medicine, 24 (4). https://doi.org/10.3126/nje.v9i3.25805
- Aryal, N., Regmi, PR., van Teijlingen, E., Simkhada, P., Adhikary, P., Bhatta, YKD., Mann, S. (2016) Injury and Mortality in Young Nepalese Migrant Workers: A Call for Public Health Action. Asian-Pacific Journal of Public Health 28(8): 703-705.
- Aryal, N., Regmi, PR., van Teijlingen, E., Dhungel, D., Ghale, G., Bhatta, GK. (2016) Knowing is not enough: Migrant workers’ spouses vulnerability to HIV SAARC Journal of Tuberculosis, Lung Diseases & HIV/AIDS 8(1):9-15.
- Adhikary P., Keen S., van Teijlingen E. (2011) Health Issues among Nepalese migrant workers in Middle East. Health Science Journal 5: 169-75. www.hsj.gr/volume5/issue3/532.pdf
- van Teijlingen E, Simkhada, P., Adhikary, P. (2009) Alcohol use among the Nepalese in the UK BMJ Rapid Response: www.bmj.com/cgi/eletters/339/oct20_1/b4028#223451
- Adhikary, P., Simkhada, P.P., van Teijlingen E., Raja, AE. (2008) Health & Lifestyle of Nepalese Migrants in the UK BMC International Health & Human Rights 8(6). Web address: www.biomedcentral.com/1472-698X/8/6.












 BU attendance at third annual GCPHR meeting in June
BU attendance at third annual GCPHR meeting in June Interactive Tangible and Intangible Heritage Applications – BU student work featured in new book chapter
Interactive Tangible and Intangible Heritage Applications – BU student work featured in new book chapter Second NIHR MIHERC meeting in Bournemouth this week
Second NIHR MIHERC meeting in Bournemouth this week MSCA Postdoctoral Fellowships 2025 Call
MSCA Postdoctoral Fellowships 2025 Call ERC Advanced Grant 2025 Webinar
ERC Advanced Grant 2025 Webinar Horizon Europe Work Programme 2025 Published
Horizon Europe Work Programme 2025 Published Horizon Europe 2025 Work Programme pre-Published
Horizon Europe 2025 Work Programme pre-Published Update on UKRO services
Update on UKRO services European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease
European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease
Explore our work, meet our partners, and find out how you can collaborate with us by clicking here! MIHERC is led by Sheffield Hallam University, with Bournemouth University as a key partner and the important funding coming from NIHR (National Institute for Health and Care Research) Maternity Challenge Initiative. The BU key academics are: Huseyin Dogan, Vanora Hundley, Edwin van Teijlingen, and Deniz Çetinkaya. Please share with all who may be interested.