
CQR’s webpages have now migrated to the new Centres and Institutes pages of the Bournemouth University website. We are in the progress of refreshing and updating the new pages, but you can still connect to the old CQR webpages, at least for the time-being. It is here that you can find links to many of the specialisations of members including
Humanising Health and Social Care;
Novel and Innovative Research Methodologies;
Performative Social Science and Arts-led Research;
Narrative and Biographic Research
CQR News
Humanising Care, Health & Wellbeing
13-14th June 2019
 The Humanisation approach is supported by working practices which encourage connection to personal experience and research approaches which privilege subjective experience and knowing. Organised and led by CQR’s Deputy Director, Caroline Ellis-Hill.
The Humanisation approach is supported by working practices which encourage connection to personal experience and research approaches which privilege subjective experience and knowing. Organised and led by CQR’s Deputy Director, Caroline Ellis-Hill.
CQR Members presenting at the Conference include: Camila Devis-Rozental, Caroline Ellis-Hill, Chantel Cox, Clare Gordon, Karen Rees, Lee Ann Fenge, Liz Norton, and Sally Lee.
Publications
 CQR Members, Associates, and Doctoral Students are also busy writing. Below, just a taster from a range of members’ recent wide variety of methods and subject matter, now in press or about to be. CQR members come from across FHSS departments and several other BU Faculties. CQR and CEL have particular synergies around creativity in research and education. Many faculty claim membership in both Centres!
CQR Members, Associates, and Doctoral Students are also busy writing. Below, just a taster from a range of members’ recent wide variety of methods and subject matter, now in press or about to be. CQR members come from across FHSS departments and several other BU Faculties. CQR and CEL have particular synergies around creativity in research and education. Many faculty claim membership in both Centres!
Assoc. Member Lee-Ann Fenge:
Fenge, L., Oakley, L., Taylor, B. and Beer, S. (in press) The impact of sensitive research on the researcher: preparedness and positionality, International Journal of Qualitative Methods
Fenge, L., Melacca, D, Lee, S. and Rosenorn-Lanng, E. (in press) Older peoples’ preferences and challenges when using digital technology: a systematic review with particular reference to digital games, International Journal of Education and Ageing
Fenge, L. Cutts, W. and Seagrave, J. 2018. Understanding homelessness through poetic inquiry: looking into the shadows, Social Work and Social Sciences Review, 19 (3), 119-133
BU Visiting Prof Catherine Hennessy:
Hennessy, C.H. and Means, R. (2018). “Connectivity of Older People in Rural Areas”, Chapter 8 in A. Walker (ed.) The New Dynamics of Ageing, Bristol: Policy Press.
Member Camilla Devis-Rozental:
Devis-Rozental.C. (2018). Developing Socio-Emotional Intelligence in Higher Education Scholars. London: Palgrave Macmillan.
Member Jo Thurston:
Thurston, J., 2020. Opening a Door to a Private World: Using Auto/biographical Methodology to Explore Health Experience. SAGE Methods Cases.
Assoc. Member Carly Stewart:
Sparkes, A. C. & Stewart, C. 2019. Stories as actors causing trouble in lives: a dialogical narrative analysis of a competitive cyclist and the fall from grace of Lance Armstrong. Qualitative Research in Sport, Exercise and Health.
Stewart, C., Woodward, M. and Gough, R., 2019. ‘I’ve drawn, like, someone who was the world’: drawings as embodied gestures of lived yoga experience. Qualitative Research in Sport, Exercise and Health.
CQR Director Kip Jones, Member Jo Thurston, Assoc. Member Louise Oliver
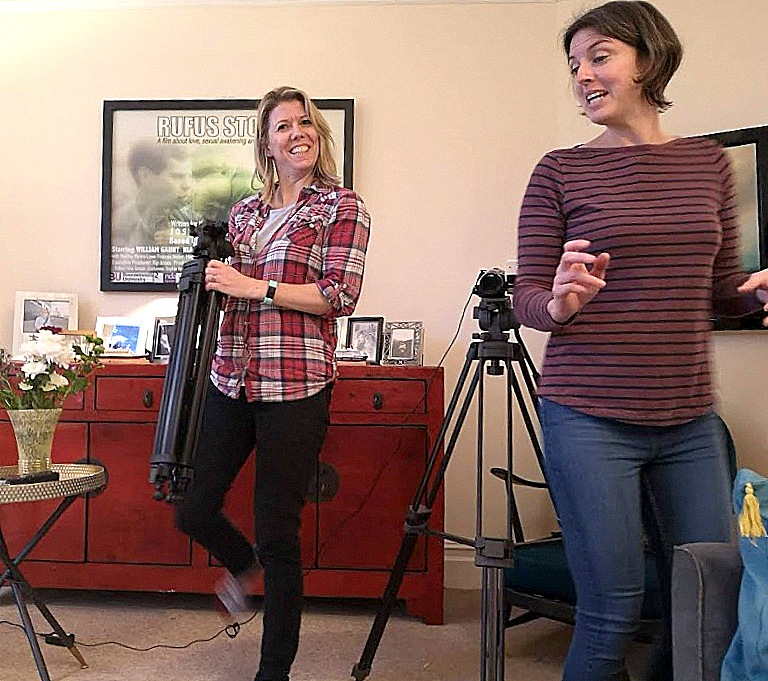
Thurston and Oliver prepare for the interview
Jones was invited by Sage Publications’ MethodSpace to write a blog article for their June/July Special Issue on Creativity. Kip transcribed his interview on biographic research conducted by CQR members, Joanna Thurston and Louise Oliver. The pair interviewed Jones, along with several other academics, for their film, “It’s not research, it’s just stories!” The film was screened at the British Sociological Association Auto/Biography Study Group Conference in December 2018. Kip Jones discusses “Biography, Auto-biography, and Creativity” in the MethodSpace blog piece.
Assoc. Member Lorraine Brown:
Kichuk, A; Brown, L; Ladkin, A 2019 Talent pool exclusion: the hotel employee perspective International Journal of Contemporary Hospitality Management
Member Jenny Hall:
Crowther, A. Stephen & J. Hall (2019) Association of psychosocial–spiritual experiences around childbirth and subsequent perinatal mental health outcomes: an integrated review, Journal of Reproductive and Infant Psychology.
Assoc. Members Janet Scammell, Vanessa Heaslip, Karen Cooper
Rosser, E., Scammell, J., Heaslip, V., White, S., Phillips, J., Cooper, K., Donaldson, I., Hemingway, A., (2019). Caring values in undergraduate nurse students: a qualitative longitudinal study. Nurse Education Today.
Member Michele Board, Associate Member Vanessa Heaslip
Board, M., Pigott, L., Olive, H. and Heaslip, V., 2019. Better Together – A Day Hospital’s move towards Integrated care. International Journal of Therapy and Rehabilitation.
CQR Members Presenting and Video Conferencing
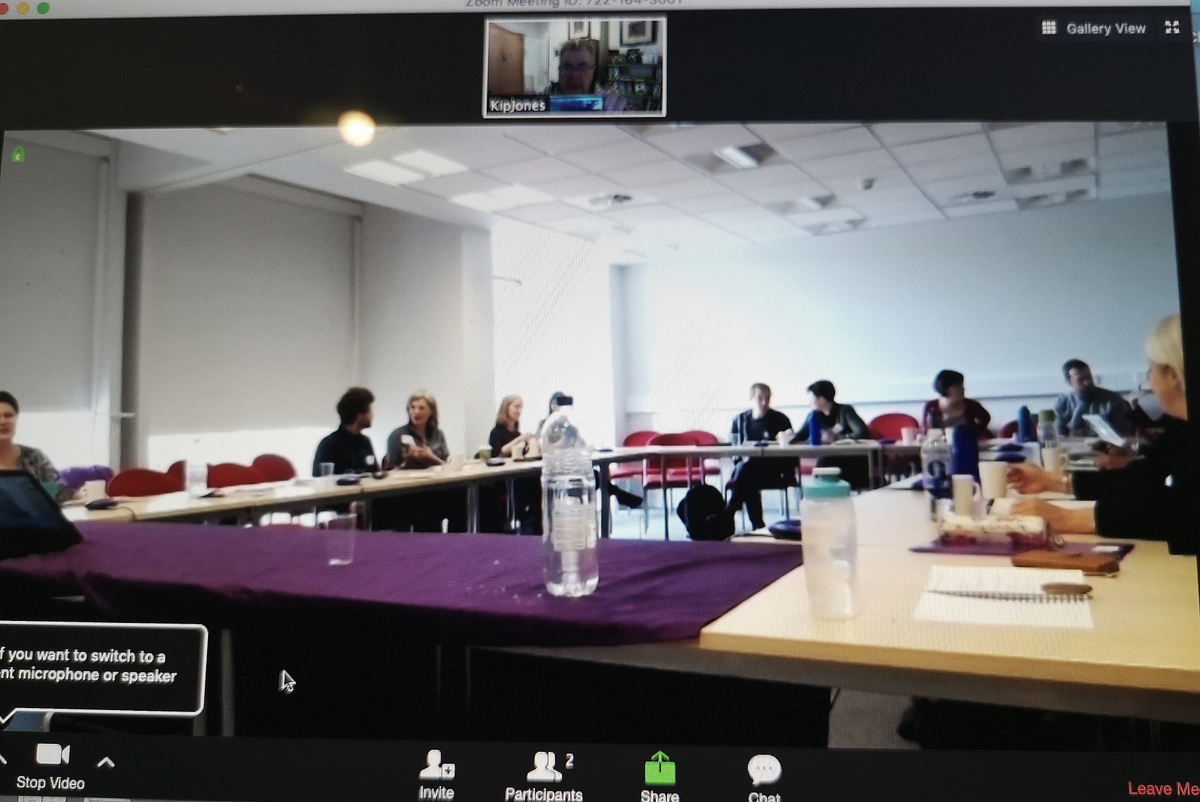
Kip Jones held a successful video session for the recent Social Fiction Conference at the Morgan Centre for Research into Everyday Lives at the University of Manchester. He will be conducting another session via video link with postgrad students at Nazarbayev University Graduate School of Education in Kazakhstan in a few weeks’ time. Both sessions centre around the award-winning short film, RUFUS STONE and Jones’ part in creating it.
CQR Deputy Director Caroline Ellis-Hill:
Ellis-Hill C, Lamont –Robinson C & Galvin K (2019) Sustaining wellbeing after a stroke: reflections on humanising lifeworld processes within an Arts and Health group – HeART of stroke EACS conference – Sustainable Caring for Health and Wellbeing Oct 1st -3rd 2019 Åbo Akademi University, Vaasa, Finland
Paglioni M, Ellis-Hill C, Board M and Branney, J and Valentine J (2019) Exploring the experience of older people who attend a hospital … The British Society of Gerontology 48th Annual Conference: University of Liverpool 10 -12 July 2019.
Doctoral student, Charlotte Clayton, has a poster accepted for presentation about her PhD research fort the University of Southampton conference, ‘Pregnancy, Maternity and the Self’ 21st June.
Assoc. Member Trevor Hearing presented:
“The Scholarly Studio: The Application of the Television Studio as a Performative Research Tool” at: Creative Practice Research in the Age of NeoLiberal Hopelessness 10-12 May 2018 University of Bedfordshire.
CQR members Lee-Ann Fenge, Kip Jones, Vanessa Heaslip Took part in the Charity Research Showcase at Bournemouth U.

Participants discussed their research with the charity sector and a wide range of charity partners.
Ideas, Ideas, Ideas!
 Following on from yet another successful year of CQR Lunchtime Seminars, it is time now for CQR members, Associate Members and Doctoral Associates, to be thinking of ideas for seminars for the next academic year. The theme for the year will be: “Methods to Our Madness!” Informal talks followed by interactive discussions are the order of the day!
Following on from yet another successful year of CQR Lunchtime Seminars, it is time now for CQR members, Associate Members and Doctoral Associates, to be thinking of ideas for seminars for the next academic year. The theme for the year will be: “Methods to Our Madness!” Informal talks followed by interactive discussions are the order of the day!
There certainly will NOT be time to explain a whole research method! Instead, presenters are asked to informally talk about how they decided on a method for a piece of research, and perhaps how that worked out (or not!) for them. CQR audiences are particularly interested in what we might call the application of ‘Creative Methods” in research!
CQR members are asked to submit ideas now as it takes time to organise the calendar for these ahead of time. Please send your thoughts via email to Kip.

 University centre. This is illustrated in each newsletter which always has sections on the three segments of Fusion: Education, Practice and Research.
University centre. This is illustrated in each newsletter which always has sections on the three segments of Fusion: Education, Practice and Research.

 The SAIL research team are working with local authorities, businesses, charities and older people to learn from experiences and to try out different strategies and is comprised of team members from the Netherlands, Belgium, France and the UK.
The SAIL research team are working with local authorities, businesses, charities and older people to learn from experiences and to try out different strategies and is comprised of team members from the Netherlands, Belgium, France and the UK.
 The Hyksos Enigma: Researching the 15th Dynasty of Ancient Egypt
The Hyksos Enigma: Researching the 15th Dynasty of Ancient Egypt
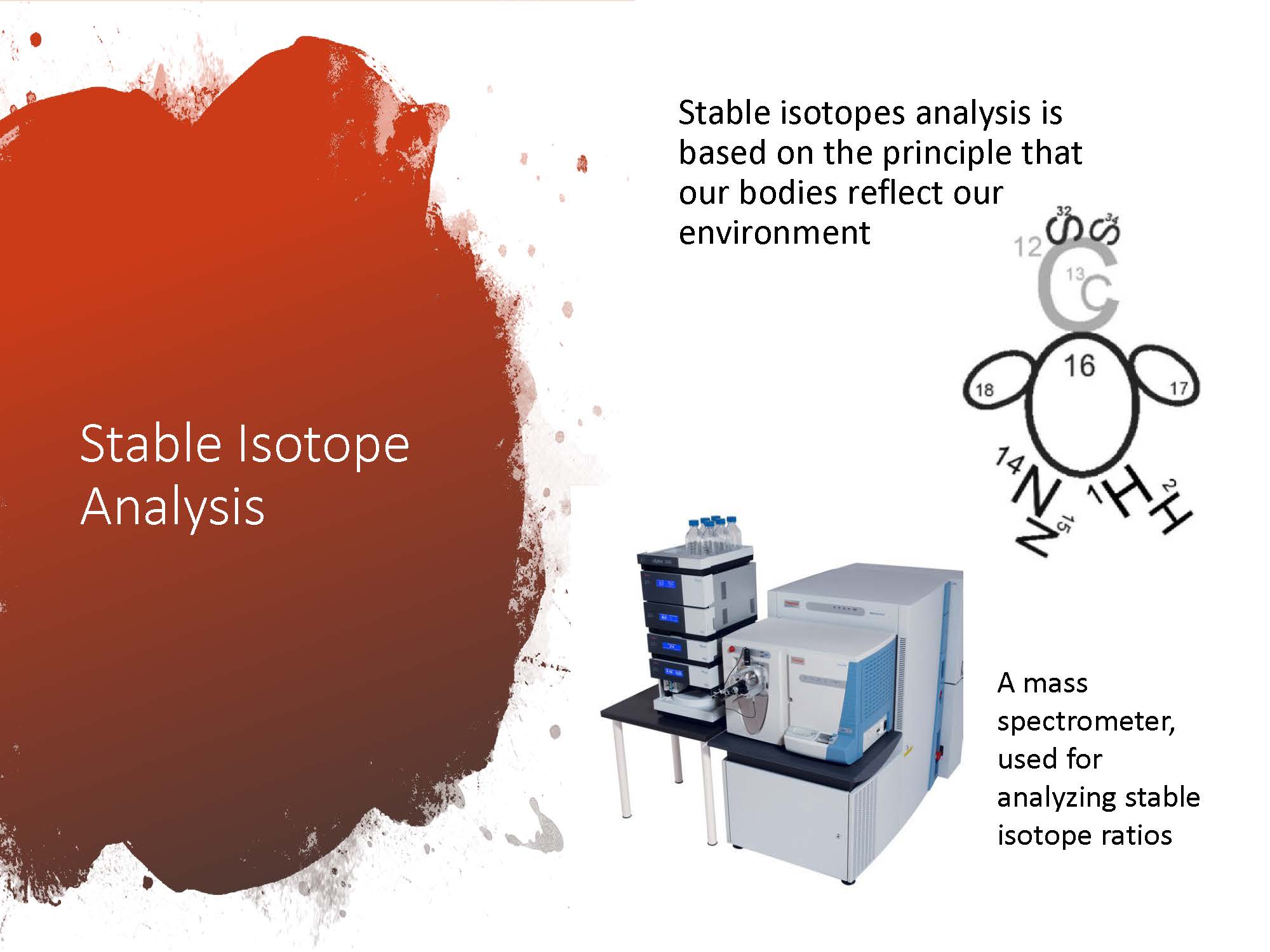

 Bournemouth University’s Public Lecture series aims to offer BU academics the opportunity to share some of the fantastic research they’ve been working on with members of the public. Allowing a diverse and enthusiastic audience to ask lots of questions and engage in interesting discussion about the research taking place here at Bournemouth University.
Bournemouth University’s Public Lecture series aims to offer BU academics the opportunity to share some of the fantastic research they’ve been working on with members of the public. Allowing a diverse and enthusiastic audience to ask lots of questions and engage in interesting discussion about the research taking place here at Bournemouth University.





 The Humanisation approach is supported by working practices which encourage connection to personal experience and research approaches which privilege subjective experience and knowing. Organised and led by CQR’s Deputy Director, Caroline Ellis-Hill.
The Humanisation approach is supported by working practices which encourage connection to personal experience and research approaches which privilege subjective experience and knowing. Organised and led by CQR’s Deputy Director, Caroline Ellis-Hill. CQR Members, Associates, and Doctoral Students are also busy writing. Below, just a taster from a range of members’ recent wide variety of methods and subject matter, now in press or about to be. CQR members come from across FHSS departments and several other BU Faculties. CQR and CEL have particular synergies around creativity in research and education. Many faculty claim membership in both Centres!
CQR Members, Associates, and Doctoral Students are also busy writing. Below, just a taster from a range of members’ recent wide variety of methods and subject matter, now in press or about to be. CQR members come from across FHSS departments and several other BU Faculties. CQR and CEL have particular synergies around creativity in research and education. Many faculty claim membership in both Centres!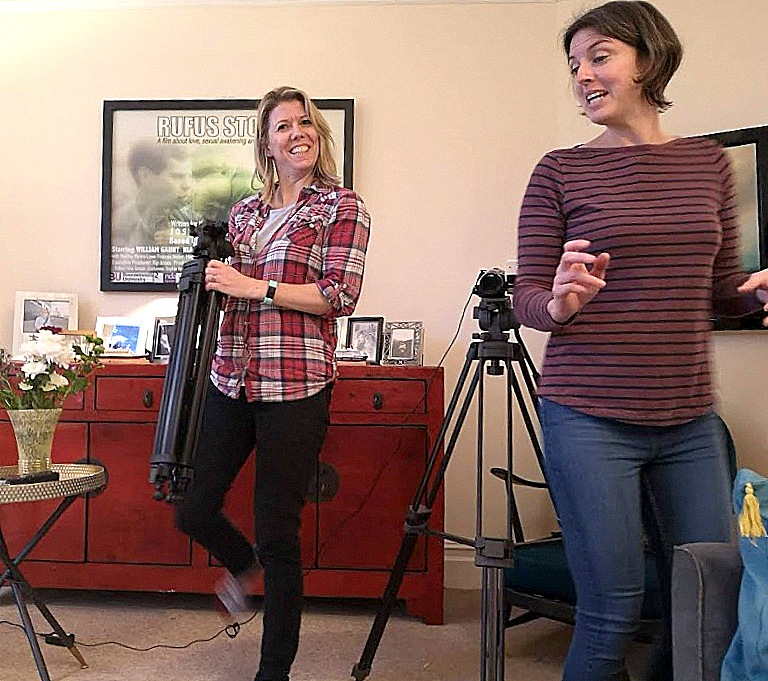


 Following on from yet another successful year of CQR Lunchtime Seminars, it is time now for CQR members, Associate Members and Doctoral Associates, to be thinking of ideas for seminars for the next academic year. The theme for the year will be: “Methods to Our Madness!” Informal talks followed by interactive discussions are the order of the day!
Following on from yet another successful year of CQR Lunchtime Seminars, it is time now for CQR members, Associate Members and Doctoral Associates, to be thinking of ideas for seminars for the next academic year. The theme for the year will be: “Methods to Our Madness!” Informal talks followed by interactive discussions are the order of the day!


 Anya’s Café Scientifique talk covered the rise and development of British seaside piers and considered their changing roles and purpose during the last 200 years. The current challenges faced by seaside piers were also examined, including the threats facing these structures in an era of climate change, rising sea levels, and storm surges. The issue of funding and costs of piers was considered and the question of ‘who pays’ for these iconic seaside heritage assets was discussed.
Anya’s Café Scientifique talk covered the rise and development of British seaside piers and considered their changing roles and purpose during the last 200 years. The current challenges faced by seaside piers were also examined, including the threats facing these structures in an era of climate change, rising sea levels, and storm surges. The issue of funding and costs of piers was considered and the question of ‘who pays’ for these iconic seaside heritage assets was discussed.

 Anya said ‘the experience of presenting at Café Scientifique was enjoyable and enlightening. It was really insightful to hear about people’s experience of piers and how they value and engage with these structures. It was also good to see the levels of engagement and discussion on the issue of climate change and how seaside piers might be used in the not-too distant future as a source of blue energy production, meaning that the form and function of piers will continue to adapt in the 21st century’.
Anya said ‘the experience of presenting at Café Scientifique was enjoyable and enlightening. It was really insightful to hear about people’s experience of piers and how they value and engage with these structures. It was also good to see the levels of engagement and discussion on the issue of climate change and how seaside piers might be used in the not-too distant future as a source of blue energy production, meaning that the form and function of piers will continue to adapt in the 21st century’.






 The article titled “The effects of 8 weeks of inspiratory muscle training on the balance of healthy older adults: a randomized, double-blind, placebo-controlled study” has been published by Physiological Reports.
The article titled “The effects of 8 weeks of inspiratory muscle training on the balance of healthy older adults: a randomized, double-blind, placebo-controlled study” has been published by Physiological Reports.













 From Sustainable Research to Sustainable Research Lives: Reflections from the SPROUT Network Event
From Sustainable Research to Sustainable Research Lives: Reflections from the SPROUT Network Event REF Code of Practice consultation is open!
REF Code of Practice consultation is open! BU Leads AI-Driven Work Package in EU Horizon SUSHEAS Project
BU Leads AI-Driven Work Package in EU Horizon SUSHEAS Project ECR Funding Open Call: Research Culture & Community Grant – Apply now
ECR Funding Open Call: Research Culture & Community Grant – Apply now ECR Funding Open Call: Research Culture & Community Grant – Application Deadline Friday 12 December
ECR Funding Open Call: Research Culture & Community Grant – Application Deadline Friday 12 December MSCA Postdoctoral Fellowships 2025 Call
MSCA Postdoctoral Fellowships 2025 Call ERC Advanced Grant 2025 Webinar
ERC Advanced Grant 2025 Webinar Update on UKRO services
Update on UKRO services European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease
European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease