 This scheme allows universities to attract outstanding research staff by providing support for up to five years, after which time the award holder takes up a guaranteed permanent post in the university.
This scheme allows universities to attract outstanding research staff by providing support for up to five years, after which time the award holder takes up a guaranteed permanent post in the university.
A monograph and other substantial publications are expected to result from an award, so teaching and other non-research commitments are expected to be minimal during the period of full Wellcome Trust support.
Up to five years’ support is available, providing your full salary for three years, 50 per cent in the fourth year and 25 per cent in the fifth year.
Travel expenses to attend meetings are provided for five years, but research expenses are provided for the first three years of the award only.
You must be nominated by your prospective head of department and have an undertaking from the head of the institution, vice-chancellor, principal or dean that your personal support will be taken over by the institution at the end of the award.
Support is normally available only at lecturer level, although in exceptional cases awards to senior-lecturer level may be possible.
Initial enquiries about the scheme may be made by you (the potential candidate) or a department in an institution.
These enquiries should be followed by a preliminary application from you by e-mail or post including
- an explicit statement from the head of the institution, vice-chancellor or dean demonstrating the institution’s commitment to the history of medicine field, and a statement confirming that the institution will provide 50 per cent salary costs in year four, 75 per cent in year five and full salary thereafter
- CV and full publication list
- an outline of no more than two pages of the proposed project
- a letter of support from the head of department, including a statement on your expected teaching/administrative load for the five-year period (this can be sent by separate cover)
- the approximate cost of the proposal, broken down into your salary, equipment and project running costs.
If successful, you will be invited to submit a full application.
A preliminary application must be submitted before a full application is invited.
Preliminary application deadlines are:
20 June (with a full application deadline of 1 August)
1 December (with a full application deadline of 1 February)
Contact: Grants Management – Medical History and Humanities
Wellcome Trust
Gibbs Building
215 Euston Road
London NW1 2BE, UK
T +44 (0)20 7611 8499
E mhh@wellcome.ac.uk
The RKE Operations team can help you with your application.
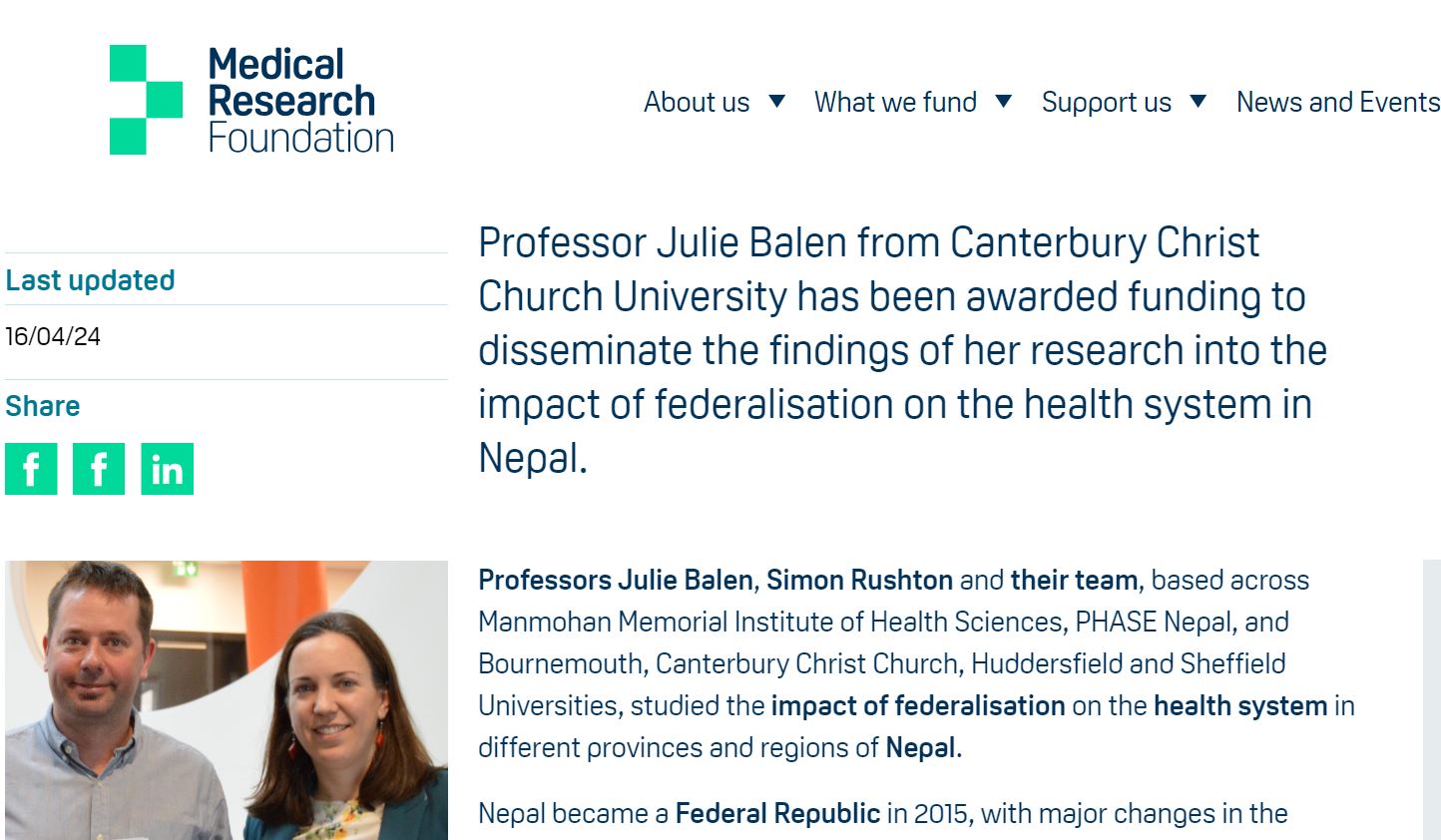 Last week the MRF announced that it has granted £30,294 for a project to ‘Strengthening Nepal’s health systems’. This Dissemination Award has been offered to expand the reach and impact of our recently completed study which was funded by the UK Health Systems Research Initiative [Grant ref. MR/T023554/1]. In this larger Nepal Federal Health System Project we studied the effects on the health system of Nepal’s move from a centralised political system to a more federal government structure in 2015. This interdisciplinary project was led by the University of Sheffield in collaboration with Bournemouth University, the University of Huddersfield, Canterbury Christ Church University and two institutions in Nepal: MMIHS (Manmohan Memorial Institute of Health Sciences) and PHASE Nepal.
Last week the MRF announced that it has granted £30,294 for a project to ‘Strengthening Nepal’s health systems’. This Dissemination Award has been offered to expand the reach and impact of our recently completed study which was funded by the UK Health Systems Research Initiative [Grant ref. MR/T023554/1]. In this larger Nepal Federal Health System Project we studied the effects on the health system of Nepal’s move from a centralised political system to a more federal government structure in 2015. This interdisciplinary project was led by the University of Sheffield in collaboration with Bournemouth University, the University of Huddersfield, Canterbury Christ Church University and two institutions in Nepal: MMIHS (Manmohan Memorial Institute of Health Sciences) and PHASE Nepal. 















 Expand Your Impact: Collaboration and Networking Workshops for Researchers
Expand Your Impact: Collaboration and Networking Workshops for Researchers Visiting Prof. Sujan Marahatta presenting at BU
Visiting Prof. Sujan Marahatta presenting at BU 3C Event: Research Culture, Community & Can you Guess Who? Thursday 26 March 1-2pm
3C Event: Research Culture, Community & Can you Guess Who? Thursday 26 March 1-2pm UKCGE Recognised Research Supervision Programme: Deadline Approaching
UKCGE Recognised Research Supervision Programme: Deadline Approaching ECR Funding Open Call: Research Culture & Community Grant – Apply now
ECR Funding Open Call: Research Culture & Community Grant – Apply now ECR Funding Open Call: Research Culture & Community Grant – Application Deadline Friday 12 December
ECR Funding Open Call: Research Culture & Community Grant – Application Deadline Friday 12 December MSCA Postdoctoral Fellowships 2025 Call
MSCA Postdoctoral Fellowships 2025 Call ERC Advanced Grant 2025 Webinar
ERC Advanced Grant 2025 Webinar Update on UKRO services
Update on UKRO services European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease
European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease