

Introduction
The four main HE funding bodies in the UK believe that ‘the outputs of publicly funded research should be freely accessible and widely available.’ The REF2021 Open Access Policy was introduced as a requirement for the next REF and it states that – all journal articles and conference contributions (with ISSN) accepted for publication from 1 April 2016 and published on or before 31 December 2020 must comply with the policy to be eligible for submission to the REF.
What does this mean?
Any non-compliant outputs that do not satisfy the policy requirements will NOT be eligible for the next REF.
What are the policy requirements?
- The outputs must be available open access (via the gold or green open access routes), three months after their acceptance date;
- The outputs must be discoverable through search engines on the internet, and free to download
- The outputs must also be in a format where they allow anyone with internet access to search electronically within the texts, to read and to download them
What does this mean to you at BU?
Once you’ve received an official notification from your publisher that your manuscript has been accepted, you should take action right away!
First of all, you should ensure that the publication record is created in BRIAN – Bournemouth Research Information and Networking, clearly specifying the acceptance date. Once you’ve created a record, following instructions on the screen, click on the BURO deposit page as shown below –
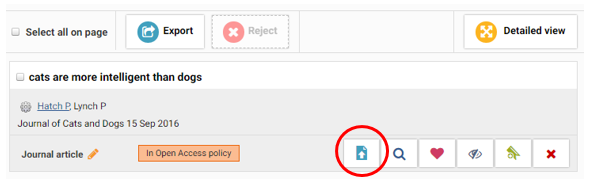
To comply with the REF Open Access Policy, you only need to upload/deposit the final accepted peer-reviewed manuscript (and not the final published version). However, depending on individual publisher copyright and policies, this is not always the case. To verify the publisher copyright policies and to decide which version of your manuscript you should use, you can do so through the SHERPA RoMEO online resource, which is a reliable source of information recommended by Research England.
Some of these deposited manuscripts may also be subjected to a period of embargo before they can be made available. Again, this would depend on the publisher copyright policies, which you can also check out on SHERPA RoMEO.
On the ‘Deposit’ page in BRIAN, you will see this message –
BURO, which stands for Bournemouth University Research Online is the University’s Institutional Repository. All manuscripts uploaded on BRIAN will be deposited in BURO and are available to anyone in the world with internet access (subject to embargo).
BURO is supported by a team of colleagues from the Faculty Library Team. The BURO team is available to support and advise you through the open access compliance process and to ensure that you are compliant with all publisher copyright and policies.
Do remember, this process has to be done within three months of your publication acceptance date! Please see this video for more guidance.
What do embargo periods mean for compliance?
As mentioned above, use SHERPA RoMEO to find out more about deposit policies and embargo periods. As long as your manuscript is deposited within 3 months of the acceptance date, the REF2021 Open Access Policy allows for an embargo period of up to 12 months for the REF Panels A & B and 24 months for the REF Panels C & D.
What if the output doesn’t meet the compliance requirements?
In some circumstances, some outputs cannot meet the open access policy requirements due to deposit, access, technical or other issues (for more information see here). If these circumstances fall under the permitted exceptions in accordance with the REF Open Access policy, these outputs may still be submitted to the REF. If you are unsure, please seek advice and guidance from ref@bournemouth.ac.uk as early as possible.
If you have questions regarding REF2021 or Open Access compliance, please feel free to contact ref@bournemouth.ac.uk or if you have questions specific to uploading of your manuscript, please contact BURO@bournemouth.ac.uk.


















 Exploring Embodied Research: Body Map Storytelling Workshop & Research Seminar
Exploring Embodied Research: Body Map Storytelling Workshop & Research Seminar Marking a Milestone: The Swash Channel Wreck Book Launch
Marking a Milestone: The Swash Channel Wreck Book Launch No access to BRIAN 5-6th February
No access to BRIAN 5-6th February ECR Funding Open Call: Research Culture & Community Grant – Application Deadline Friday 12 December
ECR Funding Open Call: Research Culture & Community Grant – Application Deadline Friday 12 December MSCA Postdoctoral Fellowships 2025 Call
MSCA Postdoctoral Fellowships 2025 Call ERC Advanced Grant 2025 Webinar
ERC Advanced Grant 2025 Webinar Update on UKRO services
Update on UKRO services European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease
European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease