 Bournemouth University has received funding from the NIHR to support an internship for a Social Work student to seek views and perception of women from ethnic minority and migrant communities. Therefor, we are seeking volunteers to take part in a small group on-line workshop to hear from women from ethnic minority and migrant communities. They are invited to share their thoughts, insights and experiences of engaging in health research so that we can better understand what would work when conducting research with this population. This work sits within a larger NIHR-funded project that aims to reduce health inequalities for marginalised mothers and babies. BU Profs Huseyin Dogan and Professor Vanora Hundley are leading workstreams within this prestigious NIHR Maternity Disparities project over the next five years (more information about the bigger project can be found here!).
Bournemouth University has received funding from the NIHR to support an internship for a Social Work student to seek views and perception of women from ethnic minority and migrant communities. Therefor, we are seeking volunteers to take part in a small group on-line workshop to hear from women from ethnic minority and migrant communities. They are invited to share their thoughts, insights and experiences of engaging in health research so that we can better understand what would work when conducting research with this population. This work sits within a larger NIHR-funded project that aims to reduce health inequalities for marginalised mothers and babies. BU Profs Huseyin Dogan and Professor Vanora Hundley are leading workstreams within this prestigious NIHR Maternity Disparities project over the next five years (more information about the bigger project can be found here!).
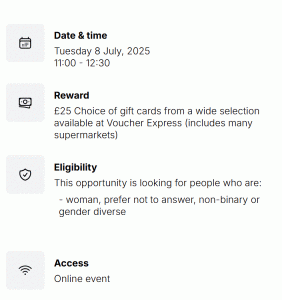
 We would like to hear from women from ethnic minority and migrant communities, also referred as women from the global majority. You do not need to be pregnant or have had a baby to participate in the workshop. If you are a woman from an ethnic minority and migrant community in the UK and you would like to take part please apply here! The event will be online on Tuesday 8th July from 11.00-12.30. No specific experience of involvement in research is required.
We would like to hear from women from ethnic minority and migrant communities, also referred as women from the global majority. You do not need to be pregnant or have had a baby to participate in the workshop. If you are a woman from an ethnic minority and migrant community in the UK and you would like to take part please apply here! The event will be online on Tuesday 8th July from 11.00-12.30. No specific experience of involvement in research is required.
Dr. Orlanda Harvey and Prof. Edwin van Teijlingen
Faculty of Health & Social Sciences
























 Dr. Ashraf cited on ‘Modest Fashion’ in The Guardian
Dr. Ashraf cited on ‘Modest Fashion’ in The Guardian NIHR-funded research launches website
NIHR-funded research launches website Academics write for newspaper in Nepal
Academics write for newspaper in Nepal New paper published on disability in women & girls
New paper published on disability in women & girls MSCA Postdoctoral Fellowships 2025 Call
MSCA Postdoctoral Fellowships 2025 Call ERC Advanced Grant 2025 Webinar
ERC Advanced Grant 2025 Webinar Horizon Europe Work Programme 2025 Published
Horizon Europe Work Programme 2025 Published Horizon Europe 2025 Work Programme pre-Published
Horizon Europe 2025 Work Programme pre-Published Update on UKRO services
Update on UKRO services European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease
European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease