
Register here
Calling early career researchers (including practice-led) for two days of sparking ideas, discovering new project partners, and developing interdisciplinary funding bids!
30 April – 1 May 2024
Bournemouth
Day 1 begins at 1230, Day 2 finishes at 1630, to enable travel from external universities.
The British Academy Early Career Researcher Network and Bournemouth University’s Centre for the Study of Conflict, Emotion, and Social Justice invite applicants for a two-day research collaboration, networking, and grant development event.
Participate in dynamic and interactive sessions to develop innovative research concepts addressing any of the United Nations Sustainable Development Goals (UNSDGs), leading to funding bids across institutions and disciplines. Your goal is to form an interdisciplinary project team and build a funding proposal in only two days.
This two-day sandpit will be supplemented with two online follow-up sessions (summer and autumn 2024) to share your project progress and experiences.
We welcome any South West-based early career (as you choose to define it) researcher, artist, practitioner or anyone with a general interest in sustainability and emerging interdisciplinary projects. You must be based at one of the South West Cluster Universities. You should be keen to work in a multidisciplinary team, and willing to commit to attending the full sandpit, on both days. No prior experience of research funding is required.
To secure your spot in the Sandpit, please complete and submit the following application – note that all participants must commit to attending both full days:
APPLY HERE BY 8 MARCH 2024: https://forms.office.com/e/AkaeieQHKx
The event is facilitated by Dr. Catalin Brylla and Dr. Lyle Skains, with advisors to be drawn from senior Bournemouth University staff based on participant disciplines and interests.
If you have any queries, please don’t hesitate to contact us.


 They presented recently published research that was included in the
They presented recently published research that was included in the





















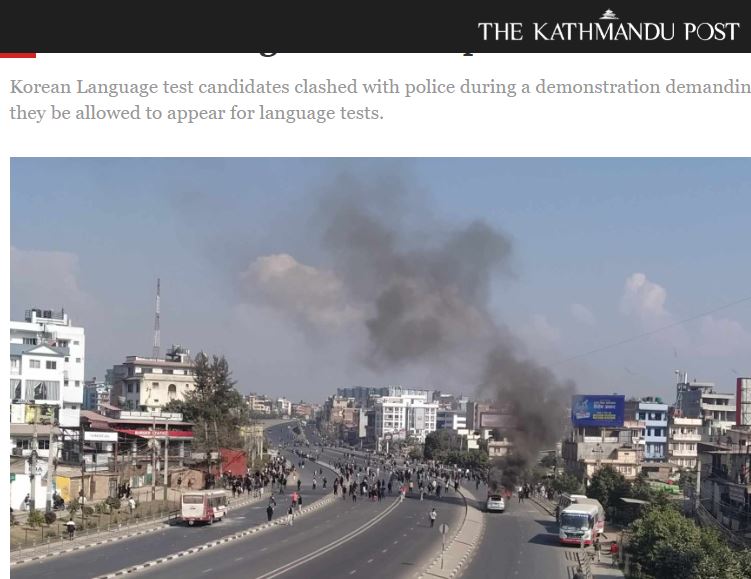













 BU academics in the news in Nepal
BU academics in the news in Nepal New CMWH paper on maternity care
New CMWH paper on maternity care From Sustainable Research to Sustainable Research Lives: Reflections from the SPROUT Network Event
From Sustainable Research to Sustainable Research Lives: Reflections from the SPROUT Network Event ECR Funding Open Call: Research Culture & Community Grant – Apply now
ECR Funding Open Call: Research Culture & Community Grant – Apply now ECR Funding Open Call: Research Culture & Community Grant – Application Deadline Friday 12 December
ECR Funding Open Call: Research Culture & Community Grant – Application Deadline Friday 12 December MSCA Postdoctoral Fellowships 2025 Call
MSCA Postdoctoral Fellowships 2025 Call ERC Advanced Grant 2025 Webinar
ERC Advanced Grant 2025 Webinar Update on UKRO services
Update on UKRO services European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease
European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease