Please see below for an update from the Health Research Authority with regard to the new system for booking in applications.
Any queries please get in touch with Suzy Wignall, Clinical Governance Advisor.
A new online booking service will be rolled out for IRAS studies on Tuesday 19 May – replacing the current Central Booking Service (CBS) telephone line. This is part of our ongoing Research Systems programme of work to improve our services for applicants.
Applicants submitting research projects through IRAS will no longer need to call the Central Booking Service to book a Research Ethics Committee, or to enable IRAS Form submission. Instead applicants will access the new online booking service via IRAS to book their application for review. The service is quick and easy to use and, unlike the current Central Booking Service, will be available 24 hours a day, seven days a week, making it easier for research applicants. If you need help and support with the new system you can call 0207 104 8008 between 8.30am and 4.30pm Monday to Friday.
In order to make use of this new functionality, applicants will be directed to a new part of IRAS which hosts the online booking service. A separate login will be required, but support will be provided. You will need to set up a new login and password for this area unless you already have a login for a NIHR system or as part of the Combined Ways of Working pilot (CWoW) pilot. In this case you can use your existing log in details.
Applicants will need to answer a series of questions online before being able to book a slot. This directs the applicant to the appropriate REC. The questions will be familiar to anyone who has used the CBS. Once you have completed your online REC booking, you will still need to electronically submit your application in IRAS using the normal process.
Applicants making contact about fast-track COVID-19 studies, should continue to follow our current guidance or email fast.track@hra.nhs.uk, DO NOT use the online booking service.
The work to build the online booking service began before the current COVID-19 pandemic. It is being rolled out now so that the system can support research applicants with non-COVID-19 studies.
Training and guidance will be available via the IRAS website. You can also watch a short video to see how to use the online booking service.

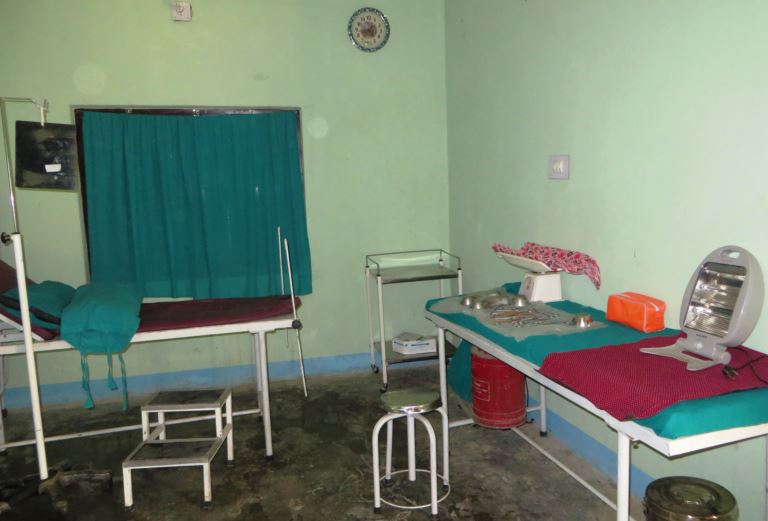




 The international social science publisher SAGE published a new textbook this week under the title
The international social science publisher SAGE published a new textbook this week under the title w weeks, Parliament has seen a surge in need for access to research expertise as it engages with the COVID-19 outbreak.
w weeks, Parliament has seen a surge in need for access to research expertise as it engages with the COVID-19 outbreak.














 BU attendance at third annual GCPHR meeting in June
BU attendance at third annual GCPHR meeting in June Interactive Tangible and Intangible Heritage Applications – BU student work featured in new book chapter
Interactive Tangible and Intangible Heritage Applications – BU student work featured in new book chapter Second NIHR MIHERC meeting in Bournemouth this week
Second NIHR MIHERC meeting in Bournemouth this week MSCA Postdoctoral Fellowships 2025 Call
MSCA Postdoctoral Fellowships 2025 Call ERC Advanced Grant 2025 Webinar
ERC Advanced Grant 2025 Webinar Horizon Europe Work Programme 2025 Published
Horizon Europe Work Programme 2025 Published Horizon Europe 2025 Work Programme pre-Published
Horizon Europe 2025 Work Programme pre-Published Update on UKRO services
Update on UKRO services European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease
European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease