Earlier this year the International Journal for Creative Media Research (IJCMR) published a journal article by Annie East, Deputy Head of Media Production Department at BU, on ways students make meaning of the risk assessment process on their undergraduate filmmaking degree. Based on Annie’s doctoral pilot study findings, this article, whilst written in a pre-covid19 environment, has 5 areas for consideration of health and safety going forward in a Covid-19 student fieldwork context. Below Annie considers how we conceive health, safety and risk before outlining 5 points.
What is safe? The social construction of safety
Safety is a subjective, constructed and socially derived notion. The Health And Safety Executive literature does not define what safety is, leaving companies and organisations to interpret or translate how that applies to their practices. Similarly risks are ‘selected’ and ‘risk is only what people choose to say it is.’ As for health, we follow current advice in how to understand what is ‘good health’.
To give more clarity we could consider the terms ‘health’ and ‘safety’ from within the industrial context in which they are being used. Since my research is about filmmaking (in an HE context), when we refer to safety at work we can consider a film set in a studio; a lighting electrician may fall off a ladder that isn’t secure and this is a result of non-safety, or ‘unsafe-ness’. When we talk about health we can view the same studio where a set designer is carrying heavy props and as a result of that act, potentially, over time, this will create health problems, linked to heavy lifting, for that person. Safety is therefore constructed by us with an immediacy, whether perceived as safe or unsafe, and health is constructed as more removed from the act, alluding to future constructs of ailment/s within the body (or mind).
So with a socially constructed definition of health and safety the linked article can be read, taking into account the added consideration of working practice and Covid19 outlined in 5 points below.
1. VR Elicitation
In the article I propose a new research method; VR elicitation. A two-tier practice of placing a 360-degree camera into fieldwork (in this case a student film shoot) and then viewing it back as a way of deepening reflective and reflexive practice for both educator and student through an immersive environment. In response to innovation around education during Covid-19, VR elicitation could be utilised to enhance, learning for students who may not be able to engage as fully with fieldwork. This would be through remote learning ‘in the round’ with peers and educators taking advantage of the immersive environment. Working with apps that can download onto smartphones and be slotted into a £30 VR visor.

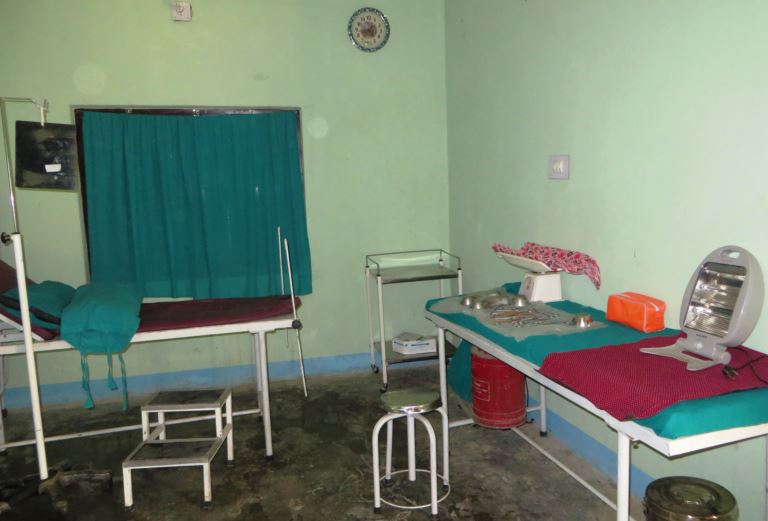
Image 1: Student film shoot

Image 2: Re-immersion back into film shoot; VR elicitation
2. The paradox within HE
The article highlights the paradoxical nature of working in a tripartite environment; education that teaches industry practice whilst complying with HE rules. With the extra layer of Covid-19 risk management incorporated into our health and safety practices, it is worth fully understanding the paradox presented within the article.
3. Risk as imagined, risk as performed
Following David Borys, I conceived the risk assessment in two steps; risk as imagined (the writing of a risk assessment) and risk as performed (the performance of the risk assessment in action). The literature acknowledges a lack of emphasis on risk as performed in scholarly research discoveries or, if it does, it discovers performance as being different to that as imagined.
4. Working beyond bureaucracy in risk management
The article posits holistic ways to approach risk management that involves engaging HE students more thoroughly. Moving us away from purely bureaucratic tick box exercises of writing a risk assessment towards a shared ownership of risk management strategy or otherwise referred to as ‘institutional magic’ by Patrick Brown. This holism is essential now that we are dealing with an invisible risk.
5. VR elicitation study findings
The pilot study teases out some of the ways students inherently keep themselves safe and are examples of where the imagined is very different to the performance. This reminds us of the importance of developing shared ownership of managing risk rather than staying purely with top-down implementation that is tied to institutional and legal power structures.
Moving forward it will be interesting to see if the increased scrutiny on Covid-19 health & safety risk management within HE results in safer student practice on a film location (or other generic fieldwork) or whether increased scrutiny on Covid-19 results in a lowering of the other health & safety practice principles.
Full linked article here.
Contact: Annie East, Deputy Head Media Production Department, Faculty of Media and Communication. aeast@bournemouth.ac.uk
Doctorate via Centre of Excellence for Media Practice (CEMP).






















 From Sustainable Research to Sustainable Research Lives: Reflections from the SPROUT Network Event
From Sustainable Research to Sustainable Research Lives: Reflections from the SPROUT Network Event REF Code of Practice consultation is open!
REF Code of Practice consultation is open! BU Leads AI-Driven Work Package in EU Horizon SUSHEAS Project
BU Leads AI-Driven Work Package in EU Horizon SUSHEAS Project ECR Funding Open Call: Research Culture & Community Grant – Apply now
ECR Funding Open Call: Research Culture & Community Grant – Apply now ECR Funding Open Call: Research Culture & Community Grant – Application Deadline Friday 12 December
ECR Funding Open Call: Research Culture & Community Grant – Application Deadline Friday 12 December MSCA Postdoctoral Fellowships 2025 Call
MSCA Postdoctoral Fellowships 2025 Call ERC Advanced Grant 2025 Webinar
ERC Advanced Grant 2025 Webinar Update on UKRO services
Update on UKRO services European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease
European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease