Welcome to your bumper edition of the weekly policy update. There has been so much action this week we delayed sending it to you twice! International Education is on the agenda with a push to the Government to do more. The Social Mobility Commission find the Government lacking and push them to make progress. It was decision time for prospective HE students all over the UK this week. The first Graduate Outcomes data has been published. REF dates have been announced and the OfS have been busy, busy, busy!
Ministerial News
Universities Minister Michelle Donelan was interviewed by HEPI. She spoke of the transition to online learning, the challenges faced by students, and launched Student Space a mental health collaboration (more on this below).
The Minister responded to questions on Black Lives Matter, disgruntlement that the student numbers cap has been applied to English domiciled students at devolved universities, delivering safe student accommodation through the pandemic, international students, graduate labour market and post graduate study. Donelan also reassured that the Augar report hadn’t been lost and that she had been working ‘extremely hard on our response to Augar’ reconfirming it would be released in line with the Spending Review and reveal ‘our vision for the sector’. She was questioned on her opinion of OfS contradictory messaging during the pandemic and responded by stating that OfS had been excellent in their feedback to the sector and in the way they shared good practice and recent guidance e.g. on consumer rights. She particularly recognised the OfS’ flexibility on Access and Participation plans and the focus on bolstering support for disadvantage that they have emphasised throughout the pandemic.
Michelle also confirmed she was working on a domestic alternative to Erasmus to ensure there was no break in provision if Brexit outcomes mean the UK cannot continue to participate in the Erasmus scheme. Stating it gives the opportunity to be more international in focus (not just accessing a subset of counties) and to prioritise social mobility and support students from all walks of life. She called it a ‘prudent plan B’.
If you do watch the webinar the first ten minutes are rather wooden as she clearly reads from a prepared speech but her responses to the questions are far more human whilst they toe the party line beautifully (although Research Professional pick up on a misstep in her response to the student numbers cap – see below).
Student Space – £3 million has been earmarked to run the Student Space mental health service as a collaboration between Student Minds, the OfS and the Welsh HE funding council. Michelle stated is available to all students at English and Welsh universities providing access to mental health support services every day for 6 month period. She stated it has been launched to close the gaps in student support services that have become apparent through the pandemic and it will work alongside the existing services. Michelle described the aspects the service will cover as:
- Prevention support and immediate interventions for those in distress
- Therapeutic interventions & stress relievers
- Suggestions for strengthening mental health & online resources
- Peer support platforms and volunteering opportunities
Research Professional cover the mental health announcement here.
In answering a question on the student number cap being applied to the devolved nations against their wishes, seemingly against previous discussions, and without an impact assessment having been done Michelle opened herself up for criticism by stating it was the English universities calling for the cap to apply to devolved institutions too. If you watch the webinar I don’t think it is quite as dire as Research Professional report, however, here is their coverage.
Donelan stated that she worked “very closely” with the devolved administrations and had discussed the numbers cap decision with them after English institutions had called for restrictions to be applied to non-English institutions. “I think the reality was we had to introduce something that would help stabilise the system and this was something that was also called for by English institutions to make sure that we didn’t create a system where institutions in Wales or Scotland could then in effect have aggressive recruitment strategies and make the policy null and void in essence.”
Her point is if Wales or Scotland over recruited whilst the English institutions are limited on how many students they can take from an already dwindling pool it wouldn’t result in the ‘sharing’ of students that the cap is designed to achieve. Setting this aside Research Professional have been on a mission to uncover which English institutions called for the cap to be applied to the devolved nations (shorter version here). They contacted all the major mission groups and UUK – all stated it wasn’t them, with most stating they either didn’t want a Government imposed cap or didn’t agree with it covering the devolved nations.
The DfE responded to Research Professional’s (RP) investigation into which universities called for the cap stating:“It’s worth noting Universities UK called for student number controls and after considering UUK’s proposal, ministers considered that it was right and fair that these should apply to all English students, wherever they were studying.”
RP weren’t impressed and said:That’s less of a statement and more of an attempt at a correction. As our coverage and all the sources we spoke to make very clear, the original UUK proposal of 10 Aprilonly refers to voluntary recruitment practices in England and Wales, and contains specific reference to the Higher Education Funding Council for Wales as the sole arbiter of student numbers in Welsh universities. As entertaining as it was we can expect the drama to die down now.
The latest DfE publication on student number controls is here.
Adult Learning
Education Select Committee Chair, Robert Halfon, writes for The Times Red Box with We are letting our adult learning system slip into disrepair
Despite the need for world-class adult learning, we’re letting our adult offer slide into disrepair. Take part-time higher education, where the number of adults enrolling has fallen by 70 per cent since 2009-10. Employer investment in training, too, is not what it once was — the proportion of employees who receive job-related training was lower in the past four years than at any point since the mid-1990s.
But worst of all, we are not doing enough for disadvantaged individuals. Adult training, it seems, is a higher earner’s game. Those who would benefit most from training are the least likely to do it. Almost half of adults from the lowest socioeconomic groups have not received any training since they left education.
He suggests starting small with community learning centres, reenergising employer-led training including a skills tax credit for investment in lower skilled workers, and the government should nurse part-time higher learning back to health including reinstating fee grants for disadvantaged part time learners whose learning would meet the nation’s skills needs.
Halfon has spoken out about adult learning regularly and is particularly supportive of restructuring and reconsidering the funding for Further Education. Halfon’s Times piece is almost verbatim the Foreword he writes for The Centre for Social Justice (CSJ) new report: The Long Game – How to reboot skills training for disadvantaged adults which is really the news rather than Halfon’s Times article.
The CSJ publication makes 14 recommendations (pages 9-11). Those most relevant to the HE sector are:
- Consult employers, sector experts and labour market information to identify existing level 4 and 5 courses that are high quality, and formally recognise these as such. Give employers a greater role in formulating new qualifications
- Once the government has identified and accredited higher value level 4 and 5 courses, it should allow students enrolled on these courses to access the same student finance system that is available for ‘prescribed’ courses.
- Reinstate tuition fee grants for disadvantaged part-time HE learners who study qualifications that meet skills needs.
- Leverage access and participation plans to better support part-time HE learners
- Improve information flows for part-time HE learning and build a single UCAS application portal for part-time courses
- Focus part-time HE providers’ minds on childcare requirements where relevant.
- Publish return on investment data for a broader range of courses and levels, including technical and vocational routes at all levels, and formulate a value-added metric for part-time, mature learning.
- Update, and continue to refine, previous labour market analyses to provide cutting-edge data on current and likely future skills demands, and hardwire outcomes data into careers advice.
- Offer workers at high-risk of losing their jobs, including those on furlough, the chance to assess their options and retrain where suitable.
The report doesn’t tackle apprenticeships and promises a separate paper on this shortly.
Research Professional cover the report and Halfon’s calls to act.
Mature students – The Guardian have a nice article giving the perspective of mature students who realised during lockdown that returning to study would enable them to take the next step in their life. It contains a comment from Sheffield on the increase in mature applications they’ve seen as a consequence of lockdown. HEPI also have a blog – Why and how the post-Covid world could offer more opportunities for widening participation in England discussing how online and cross-institution shared activity could provide wider and richer experiences for prospective students unable to access summer schools and other access initiatives, whilst reducing the burden on individual institutions. And there is a parliamentary question from Lord Fox asking the Government whether they will encourage those who have lost work due to C-19 to develop new skills through part-time HE and on providing greater financial support for learners on shorter HE courses.
International Students
Jo Johnson continues to speak out on international students, a topic that was close to his heart during his stints as the Universities Minister. Jo was opposed to including students within the immigration targets, regularly spoke on the cross-subsidisation of research and teaching from international student fees, and a fan of the post study work visa including proposing amendments to the immigration bill to support the visas. Jo writes the foreword for a joint publication: Universities open to the world – how to put the bounce back in Global Britain.
The report states that post-pandemic the international student market will be even more competitive, reluctance to travel overseas alongside travel restrictions will mean a smaller pool of students to recruit from. The report recognises that before C-19 international student numbers were predicted to reach 10 million by 2030. While the report acknowledges the potential decline it cautions against the myriad of surveys which portend ultimate doom and gloom with the decimation of international intake. Yet states that the Government and Vice Chancellors are preparing for 50-75% drop in international numbers a significant reversal for one of the great boom businesses of the globalised economy. It continues:Any decline of this magnitude would expose real vulnerabilities in university finances and highlight the way universities have been the victim of competing and conflicting Government policy objectives in Westminster over much of the last decade. Governments have long required universities to develop additional revenue streams to cross-subsidise research… and the teaching of certain strategically-significant laboratory and studio-based subjects that cost more to deliver than the £9,250 fees paid by domestic students. They have recognised that, far from squeezing out qualified domestic students, overseas students have made viable courses and research opportunities that would otherwise not be offered.
Johnson blames the Government for the UK’s declining dominance in global education:
- For much of the last decade, however, the UK Government has run contradictory policies aimed both at increasing education exports, while at the same time managing down international student numbers in a misguided attempt to reduce overall net migration to below 100,000. This confusion and ambivalence has created a volatile and unstable policy environment which helps explain why the UK – a perennial world leader in education – has gradually seen its share of the international education market slip over the past ten years.
- How rapidly UK universities bounce back post-Covid-19 will be of central importance to the UK’s economic recovery and, in the wake of the pandemic and in anticipation of the end of the Brexit transition period, the Government is now sensibly revising its International Education Strategy… It is vital it is suitably ambitious.
The report goes on to state the new strategy’s targets are not ambitious enough – By settling for growth rates significantly slower than the global market for international students had enjoyed over the previous decade the targets tacitly accepted a steady loss in UK market share. And sets out the recommendations the Government should adopt to see the UK rise back to its top 2 ranking again:
- Offer the best post work study visa available – 4 years duration
- Transform the British Council to focus on promoting UK study. Including operating a global student mobility network (Erasmus replacement), resolving the online study conundrum and creating reciprocal recognition agreements where countries don’t currently recognised the online elements.
- Strategic push to rebalance student flow – doubling numbers from India. Including India and China in the low risk country category.
- End the hostile bureaucracy – Cut the time consuming and off-putting red tape to allow overseas students to study here increase flexibility for English testing and Tier 4 visas, holding universities to account for non-compliance.
- Mitigate the effect of international travel restrictions for international students
- Commit to the 2030 targets including UK to be second in world as overseas study destination; the International Education Champion to be accountable and report progress to Parliament annually; and education exports as central within the UK’s post-Brexit trade strategy.
The report concludes:The university…is now one of the great global institutions…For years, however, they have had their hands tied, unable fully to unleash their potential, tied down by bureaucracy, obsessions with poorly-crafted immigration targets and pettifogging rules. The moment has come to ensure that the UK’s great universities can play their full part in this next chapter of Britain’s engagement with the world beyond its shores… Seize the moment. The time for action has come.
Research Professional comment on the report:As a package of measures it is both wildly optimistic—four-year work visas, no travel restrictions—and a measured technocratic approach that would have credibility with parliament and the civil service, if not the actual government…. The problem is that the ink is still not dry on trying to get the two-year work visa over the line. But, you know, God loves a trier.
And acknowledges that the pandemic cannot be blamed for everything:Beyond the policy recommendations, the report has some telling analysis of the state of play on international recruitment. The truth is that even before the pandemic, UK market share was flatlining at a time when global demand for higher education was soaring.
ITV news also cover the report.
Parliamentary questions on international student matters this week include the Trade Minister recognising HE as an export sector and confirming the Government is exploring the extent that trade negotiations could support education services and collaboration in science, research and innovation.
Visas – On Tuesday evening the Home Office issued guidance on temporary Tier 4 concessions as a result of C-19. It confirms that international students studying online will be eligible for a visa the following academic year when they attend for in-person study once restrictions are lifted. It also confirmed access to the post-study work visa assuming they are studying in the UK by 6 April 2021. However, some countries, such as China, would not recognise the degree if it contained too much online learning. This is Johnson’s point above – that residents of other countries need their UK degree recognised by their own country – the reciprocal agreements need to be in place otherwise this is yet another discourager. There is also a response to a parliamentary question stating sufficient capacity is in place to ensure visa applications are processed in time.
The Universities Minister responds to a parliamentary question on how the Government will support universities with international students who are subject to the quarantine policy.
Other related parliamentary questions:
OfS
The OfS followed up last month’s appearance before the Education Select Committee with additional information on their HE regulatory approach during the pandemic, with particular mention of access and participation work. You can see all the follow up letters to the Committee’s inquiry here including those from other organisations.
The OfS have published their latest briefing note showcasing advice and case studies where providers have effectively supported postgraduate research students. They have also published a briefing note providing information advice and guidance for prospective students, another on student and consumer protection and one on Graduate students: getting into employment showcasing how universities work with students to help them access appropriate employment.
The OfS also held a webinar on Supporting BAME Students during the Coronavirus pandemic on 5June. You can watch the recording of the event here, see the blog here, panel sessions here, and our thanks to Ann Barnes who has shared her summary notes from the event here.
Research Professional have an article on the OfS’ response to the precarious HE situation. It covers the OfS consultation on student protection plans, their temporary powers, and regulatory action. Meanwhile Wonkhe have a completely different approach to student protection – a try before you buy approach. The blog is useful at the very least for the analysis on transferring institutions, particularly the reminder that disadvantaged students don’t transfer.
Seize the day – Admissions
EX-UCAS Chief Executive Mary Curnock Cook writes for the Times Red Box on why this isn’t the year to defer:
- Why on earth is it a good idea to defer your university application to next year when gap years will be blighted by poor job opportunities and travel restrictions? On top of that, if mass deferrals from 2020 are added to the expected intake next year, 2021 could quickly become the most competitive year to enter university in recent history. Already we know that the 18-year-old population starts to increase again in 2021 for the first time since 2009, and we could expect international student applications to be sharply up after thousands of overseas students’ plans were thwarted by the virus this year.
- Instead, 2020 looks like being the best year for university admissions due to a combination of a low point in the school-leaver population, a likely dip in international enrolments and general uncertainty because of the virus crisis. Despite the lottery of calculated exam grades, 2020 could be the year for thousands of students to step into the university place of their dreams without having to negotiate clearing or pleading calls from school to university for leniency over missed grades.
- if students enrol with an open mind, they could become part of the heroic generation which cocked a snook at tradition and were bold enough to join the experiment which will herald a new age of higher education. Do they really want another three years of classroom-style teaching? Of course they don’t. They want to be prepared for 21st-century employment where communication, enterprise and commerce take place in a digital world.
- If some social aspects of university are curtailed in the early part of the academic year, this will be a shared constraint with the rest of the population. If you’re not allowed to socialise, play sport and go to the student union bar at uni, you probably wouldn’t be allowed to had you stayed at home.
- And if it’s value for money for your £9,250 fees that’s worrying you, I’d wager that the corona cohort will look back and consider that the extraordinary circumstances in which they embarked on their higher education journey made them better students, better citizens and prepared them better for their careers than any preceding cohort. Bluntly, short-term disruption at university is a better option for most students than short-term disruption at home or a year on the job market.
Decision Time – Students had until yesterday to confirm their first choice of institution through UCAS; so the extent of deferral within the sector will be known shortly. Wonkhe report on the newly released admissions data from UCAS highlighting that despite predictions students’ are not deferring in drove (yet):
- …as of earlier this week, 31,380 applicants have at least one deferred choice, compared to 30,760 at the same point last year – a 2 per cent increase, which should be seen alongside a 1.2 per cent rise in overall applicants…UCAS COO Sander Kristel suggests that figures show that while more applicants are thinking of deferring, those thoughts are “not yet translating into definitive actions”.
Sander has a blog exploring this in more detail.
And on decisions about whether to attend HE:
- 20 per cent of respondents, up from 15 per cent a month ago, say their biggest single worry is “missing out on the experience”. This has now overtaken online learning (16 per cent) as the most common top worry about starting university. Young applicants’ other top concerns are not having access to the right facilities (10 per cent), not having enough contact hours (7 per cent, increasing from 4 per cent in early May), and not having a freshers’ week (also 7 per cent).
First to defer: The Office for National Statistics (ONS) has highlighted that students who defer entry to university are more likely to achieve a first-class degree than those who go straight from school:
- 18% of deferrers achieved a first compared to 12% of school leavers.
- 23% deferrers undertake postgraduate study compared to 15% of school leavers.
- 47% of deferrers were in high skilled work post-graduation compared to 45% of school leavers
- 46% deferrers achieved a 2:1 compared to 51% of school leavers
The ONS state the results from their Annual Population Survey are consistent across groups who graduated before, during and after the last economic recession in 2008. There doesn’t appear to be an analysis of the characteristics of the deferral group which could affect these results, e.g. if the deferral group had a higher proportion of advantaged students. ONS commentary
The Times cover the story with the header: Coronavirus gap year is the road to first-class degree.
However, Wonkhe explain that the results aren’t quite as they seem:
- This isn’t a particular cohort, just all graduates that the ONS have been able to identify – and the deferral is simply people who left school, registered as doing something (or nothing else) and then registered as a HE student….plus the fact we are comparing a very small group with a very larger one gives us a bit of a problem.
- Gap year graduates also earn less than their straight-to-uni equivalents – you’d expect the opposite, really. They are more likely to net a first, but also more likely to net a third or below. They’re less likely to hold a post graduate qualification, and less likely to be employed.
- In essence, our tiny gap year group is weird. Not in a negative way, you understand, just that as a small group they are likely to contain outliers for all kinds of reasons. So we are not so much seeing a trend as more outliers.
Accepting Independent Students
There is a Wonkhe blog on the independent students who cannot be awarded a predicted A level grade; home educated, distance learning candidates and private entries will all have to take the autumn exams to obtain a grade. But this may mean postponing entering higher education for a year – something that may be feasible for younger students considering a gap year but won’t work for older students needing to make long term decisions about their jobs and families.
The blog calls on universities to be compassionate in accepting these students:
- unless universities are flexible, very few independent students will be able to take up their conditional offers of university places this autumn. Ofqual and the awarding bodies have been aware of this problem from the beginning and have worked hard to find alternative solutions for this group, but without success.
- Universities should consider giving a place this year to a student who has a conditional offer on the basis of an academic reference or an assessment such as an essay set by the university. This could work well providing it is planned carefully and well in advance.
- We want universities to be aware that all independent students have been disadvantaged this summer and to be as understanding as they can when considering their applications.
And:
- We are looking to the future and are calling for a more flexible and resilient exam system where students have the option to be assessed at a distance, possibly using a combination of coursework and proctored exams. This would also open up a route to national qualifications for people who need special access arrangements or are institution- bound like prisoners.
Student Numbers Cap
The draft legislation on the student numbers cap has been published. (Research Professional cover it briefly here.)
Wonkhe continue to delve into the detail of the Student Numbers Cap and have come up with a new potential loophole they felt could be exploited:
- if you have the appetite to gamble and a strong expectation of underlying growth, in some ways it actually makes more financial sense to recruit six per cent above the cap this year than to stick to the rules. Sure, you may be in breach of the new ongoing condition of registration, E6, but is that really going to be important?
- This potential loophole applies particularly to large providers with diverse income streams that can be certain of sustained growth in applicant demand – for universities that have home fees as a primary source of income, the risk would probably be too great… that’s large, prestigious, research intensive providers quietly being given an incentive to grow at the expense of others – twice in the same policy is beginning to feel like more than an accident. All the signs are now that government policy is favouring one sort of university at the expense of another on a level not seen for many years. Is this really the “stability” the government wants for the sector post-pandemic?
You can read the Wonkhe article here to make up your own mind whether you agree.
Emma Hardy, Shadow FE & HE Minister, asks if degree apprenticeships are included within the student number controls.
Research
Research Integrity
UKRI have published Research Integrity: A Landscape study. It highlights the importance of personal integrity and the influence of local culture as key to research integrity, with bullying and harassment identified as the single biggest negative influence.
Key findings include:
- Almost unanimous agreement from respondents that personal integrity drives research integrity and that local culture can have a strong influence on behaviour.
- Good leadership and management, professional development, sharing research, and the opportunity to collaborate and work with colleagues from other disciplines are all considered to have a strong positive impact on research integrity.
- The report found that bullying and harassment was the biggest negative influence on integrity and almost eight out of ten respondents believe that researchers feel tempted or under pressure to compromise on research integrity at times.
- Research integrity includes the professional standards that researchers should adopt and research organisations should promote, as well as the core values of honesty, rigour, openness, transparency, care, respect and accountability.
UK Research and Innovation Chief Executive, Professor Sir Mark Walport, said:
- “Maintaining high standards of research integrity is of overwhelming importance to the UK’s research and innovation sector, and this should be re-emphasised in the current crisis.
- Research integrity is central to our vision at UKRI and cuts across all we do, as a research organisation, funder and partner. The report published today will inform our ongoing work, helping us to enhance positive incentives for research integrity and address negative ones. It will align with other activities in this area, such as the creation of a Research Integrity Committee, in supporting our commitment of ensuring a vibrant, healthy and responsible research climate. We will work with partners and stakeholders across the sector to address the issues highlighted in the report.
Dr Helen Munn has been appointed as the Interim Chair for the Research Integrity Committee, which will champion research integrity in the UK and independently examine whether research institutions have followed appropriate processes to investigate misconduct. She will lead on subject matter expertise to refine the scope of the Research Integrity Committee over the coming months before an open recruitment for the Committee Chair and members gets underway.
REF Deadlines – 31 March 2021 has been confirmed as the revised deadline for REF 2021 submission. The press release states:
- The exercise is scheduled to resume on 31 July 2020, eight months ahead of the revised submission deadline. Updated guidance on any revisions to the exercise will be published by this date, following a further period of engagement with the HE sector and wider partners. As previously advised the staff census date of 31 July 2020 remains unchanged.
- In recognition of ongoing uncertainty about the effects of COVID-19, the funding bodies have set a review date in November 2020. This will consider the level of disruption being experienced, and whether further contingency arrangements for the REF may be necessary.
There will be an extension to the impact period until 31 December 2020 to enable case studies affected by, or focusing on the current response to, COVID-19 to be assessed in REF 2021.
The decisions have been made following the March 2020 consultation which the REF Steering Group Chair confirmed received a good response and a clear preference for the March deadline. Here is the summary of the consultation responses.
The Panel nomination process is expected to be confirmed shortly.
ARPA – The Commons Science and Technology Committee are running an inquiry into the nature and purpose of the proposed ARPA style new research funding agency. ARPA aims to fund emerging fields of research and technology with longer term more sustainability funding targeted at high-risk high-pay off blue skies science, engineering and technology research innovation. The Government confirmed their intention to fund this agency in the Queens Speech following the December 2019 election. It is estimated it will cost £800+ million. The Committee are questioning how it might address current gaps in the research system, the implications it will have on the existing funding agencies, whether it should be based outside of the golden triangle, how to maxmimise ARPA effectiveness and learning from other countries.
At PMQs last Wednesday Boris reconfirmed support for the Advanced Research Projects Agency confirming funding of £800 million:
Alan Mak (Conservative): Britain’s new Advanced Research Projects Agency is vital to securing our status as a science and technology superpower, particularly as we recover from coronavirus. Will my right hon. Friend commit to protecting its funding and its independence, so that there are no obstacles to delivering transformational breakthroughs from clean energy to new vaccines? [903040]
Boris Johnson (Conservative, PM): Yes, and I thank my hon. Friend; he is absolutely right. We will be funding the Advanced Research Projects Agency to the tune of £800 million, and it will be tasked with supporting really revolutionary breakthroughs in this country. It is the UK—from the splitting of the atom to the jet engine to the internet—that has led the world in scientific research, and under this Government we intend to continue.
Erasmus+
Michelle Donelan responded to the parliamentary question on the timescale for an alternative Erasmus+ scheme post-Brexit confirming that the Government remains committed to international exchanges in education, that current exchanges and research bid funding remains valid for the original full time period (it won’t be axed early due to Brexit) and that:Beyond the 2020/21 academic year, the government remains open to considering participation in elements of the next Erasmus+ programme, provided that the terms are in the UK’s interests. Future participation is subject to our ongoing negotiations with the EU. In parallel, the government is continuing to develop the option for a domestic alternative to Erasmus+, to ensure that we are prepared for every eventuality, and will publish information on a possible alternative, if appropriate, in due course.
Other parliamentary questions include confirmation the Research Sustainability Taskforce won’t get involved in UKRI’s Open Access review nor will it consider the financial effects of the review’s outcome for the HE sector. That the Taskforce is meeting fortnightly.
Funding & Data
The Office for National Statistics have published the 2018 R&D expenditure figures covering research councils, HE funding councils and Government departments.
Science Minister Amanda Solloway announced 38 projects allocated a share of £70 million for quantum technology projects.
Lord Callanan confirmed the financial support the Government are providing for AI research.
The House of Commons Library have the latest briefing on R&D spending.
Gender inequality amelioration
Research Professional report on a Spanish science unit who are trying to find ways to limit the impact of the lockdown on female researchers. It has been reported that women are submitting fewer funding applications and journal articles during lockdown compared to men. This has been attributed to women taking on increased responsibilities for childcare, home schooling and domestic duties. The Spanish team call on funders to factor this into the design and requirements of their future funding calls and for research institutions to assess their career support policies ensuring they promote gender equality.
The Royal Society of Chemistry are working with publishers of peer reviewed journals to proactively tackle gender bias. Previous research demonstrated that a publishing bias does exist and the Royal Society has committed to addressing this.
Public Perception of Universities
Last week we mentioned the Public First survey which aimed to establish the public’s perception of the importance of universities, particularly in recovering from the pandemic. This week Wonkhe managed to access the underlying data and highlight public opinion that Public First didn’t publicise.
- 17% per cent think that universities should benefit from increased public spending (compare 83% for the NHS)
- Only 37% feel that social work should be a graduate profession.
- Only around 1% feel that universities should be expanded post-Covid-19.
- 93% do not feel that supporting more people to go to universities should be in the government’s top five priorities. Note – the blog explains how this last question wasn’t really a fair comparison due to the other possible responses to the question.
It also critiques the survey design overall ironically quoting the founder partner of Public First who states:Using research to consider how best to sell an approach to the public is … generally seen as being a much more appropriate way of using research. This is not about coming up with specific policy ideas and positioning, but working out the best way of framing an issue to the public – working out the language that should be used to describe an issue and how to present it overall. This really matters. Express a police idea in one way and people hate it; change the language and they like it
Wonkhe say:The idea of these tables circulating without caveats in high-level policy circles is worrying, and one that could easily have been avoided by asking different questions…the sector could have missed out on the opportunity for a deeper conversation about how the public really perceives universities. Meanwhile, we know that this government doesn’t ignore opinion polls and probably won’t ignore the findings of this one either.
Social Mobility
The Social Mobility Commission is dogged by the frustrations of making little progress in tackling systemic social inequalities. The last Commission resigned en masse in protest at the lack of progress and their belief the Government wasn’t doing or committed enough to the agenda. The new Commission has lost its leader who was unable to juggle the role alongside her substantive employment during the C-19 crisis. And the new Commission’s report describes similar frustrations with Government departments failing to act.
The new report Monitoring social mobility 2013-2020: Is the government delivering on our recommendations describes the Commission’s role as a critical friend of Government holding it to account for its efforts to create a more socially mobile society, while also offering a longer perspective, free from short-term political cycles. It finds a mixed picture when examining whether the Government departments have adopted the Commission’s 52 recommendations in the last seven years. The recommendations covered:
- early years
- education
- employment
- housing
- health
- transport
They find:
- On 23% of recommendations strong progress has been made or the recommendation achieved
- On 46% some, but insufficient, progress has been made
- On 31% of recommendations little or no action occurred
Progress made (before the pandemic) was:
- more disadvantaged pupils staying in education for longer,
- more disadvantaged students going into higher education, and
- more people in work than ever before.
Of major concern the Commission notes:
- an increasing number of children growing up in relative poverty
- 600,000 more than in 2012, and set to increase further due to pandemic
- BAME children more likely to be impoverished (46%) compared 25% white British children
- a crisis in the early years’ workforce (45% of workers on tax credits or benefits), the status and prestige of the workforce must increase
- Disadvantaged attainment gaps persists (25% disadvantaged achieve GCSE English & Maths compared to 50% of all other pupils).
- The Government hasn’t acted on a 16-19 disadvantage premium, the Commission says the Government doesn’t do enough to prioritise disadvantaged young people in this age group
- The Government hasn’t done enough to reverse the declining participation in adult education and training – half of all adults from poorest background received no training after leaving school.
- Life expectancy is falling for women in the most deprived 10% of areas. Issues around health inequalities and race linked to socio-economic background have also been exposed by coronavirus (COVID-19) and need to be addressed.
Of primary concern was the lack of joined up thinking across government departments. Therefore, the report launched a call for the Government to set up a central unit and cross-Governmental common strategy to support the work of the social mobility commission and achieve greater social mobility in the UK. The unit should coordinate actions across Government and ensure the Commission’s recommendations are delivered.
The need to tackle social mobility has been exacerbated by the pandemic which is impacting the poorest groups in society most. Social mobility has never been more important. It is the poor and the young who will suffer most from the economic downturn.
Pragmatically the Commission recognise We have made dozens of recommendations over the past seven years… As we collectively deal with the shock of the pandemic, it is imperative that improvements in social mobility are integrated into the way we regroup and rebuild. We must design a recovery that ensures that the needs of the most vulnerable are met. The Commission highlights the following 8 key points to move forward in the face of declining social mobility
Page 12 shows the Government departments responsibilities and how they equate to social mobility – illustrating the call for a joined up cross-Government approach. And pages 15 onwards contain the detail of the questions asked to Government and their response on what had been implemented and progress made which enabled the judgement that one third of recommendations hadn’t been implemented. Pages 40-49 cover post-16 education, including a red – no progress – rating for understanding what works within technical education for disadvantaged learners (despite the Government’s push to increase the status of technical routes and the accelerated rolling out of T levels).
The BBC cover the story.
HE Sector Financing
UCU have launched their Fund the Future campaign. Urging for support funding for HE institutions and requesting a meeting with PM Boris. Research Professional (RP) cover the campaign here but the real analysis is contained in the RP playbook blog here. Excerpts:
- As a request directed at the government, the UCU campaign is similar to the Universities UK bailout terms, which were roundly rejected by the Treasury and Downing Street… Like the UUK request, this campaign also asks for financial help without expecting to give anything back in return. If that strategy did not work for vice-chancellors, it certainly is not going to work for the trade union… During the online launch of the campaign, Grady also placed an emphasis on opposing cost-cutting measures in universities… There are only two possible responses by vice-chancellors to the scale of this crisis: cutting expenditure or going bankrupt…
- But what would work as a campaign that might move the dial on government inaction over financial help for higher education?
- First, make alliances with others outside universities: not just further education colleges but the NHS and schools… A request to government that is likely to be well received would be: We will do whatever it takes to ensure the education of Britain’s young people, in return for a long-term underwriting of the finances of all educational settings as a contribution to the national recovery. That means giving ground on engaging with a return of pupils and students to schools and campuses, while doing everything that needs to be done to keep staff and their charges safe… It ought to be clear by now that the government has no real plan or special interest in the plight of schools, colleges and universities… Together, education leaders and unions need to get out in front of this narrative of paralysis, take charge of their own professions, provide confidence to students and shame the government into action.
- They will find plenty of sympathy in the country from frustrated parents and bored young people. If this populist government reacts to one thing, it is popular opinion. Universities should make common cause with schools and propose a long-term investment package in return for a mass effort to unlock education.
Student Finance
The Student Loans Company has announced an extension to the application deadline for student loans for full-time returning students to 30 June. They have also opened the applications for part time and post graduate master’s students tuition and maintenance loans.
Racial and Ethnic Disparities within HE
Valerie Amos, outgoing Director of SOAS moving to become Master of University College Oxford in September, writes for Research Professional in A case to answer to describe the persistent racial and ethnic disparities within HE. She states
- initiatives are not enough. We have a case to answer. We are not all bastions of inclusivity. Our students tell us that all the time. Culture change is tough and takes time. Race equality is not a bolt-on or a luxury…
- I have spent a substantial part of my working life campaigning for equality and social justice. I have sat on committee after committee, making recommendation after recommendation about what needs to change. I am not naive. I don’t think the kind of transformative change needed can be delivered by universities on their own. It’s a responsibility for all leaders in every institution in the UK. But this is a moment, potentially, of profound change in the public mood. We’ve wasted chances like this before. We mustn’t waste this one
The Black British Academics network have also published a new collection of essays on racial inequalities: Transforming the Ivory Tower: Models for gender equality and social justice. The network is led by BU’s Deborah Gabriel.
New commission: The Government has set up a new commission on race and ethnic disparities. In relation to the Commission the Minister for Equalities, Kemi Badenoch, stated Education is seen as a key social mobility lever.
Graduate Outcomes
The data from the first Graduate Outcomes statistics batch has been released! It features the outcomes from the 2017/18 student cohort. You’ll recall that the Gradate Outcomes survey replaced the DLHE (student destination data) covering students 15 months post-graduation and while we’ve had the LEO (Longitudinal Education Outcomes) data to play with there has been a transition hole. This week it is the sector level data that has been released with provider level data more widely available next week and further analysis scheduled for release on 9 July. The data is considered ‘experimental’ with HESA openly acknowledging that it will be tweaked and refined in future years to achieve a robust survey that will command the confidence of the sector. There has been lots of commentary on caution around the results and the new measures introduced.
Research Professional state: This year’s survey is described as “experimental” because it is new and still undergoing evaluation. HESA stresses that this “does not imply that the statistics or source data are of poor quality, nor does it provide any signal to suggest that the data is not fit for any operational purposes”.
They go on to note the response rate was only 47% (HESA set a 60% response target initially) acknowledging the rate would be lower because it is longer after students graduate, however they consider the number involved as disappointing and are concerned about TEF implications.
Wonkhe describe the enhancements for this data set:…there’s more nuance: for the first time, graduates can now identify themselves as a carer, as self-employed, or as travelling. Data quality should be better now we have moved to centralised collection and coding. By far the most interest will be in some of the more reflective questions, which ask graduates to put their university experience in context with the meaningfulness of their current work and future plans.
Alongside fair warning: Graduate Outcomes would need – almost by default – to feature in a revamped TEF and in other regulatory activity, but it will take a while to build up the kind of time series that would be useful. The need to understand data collected during and beyond the Covid-19 pandemic to a rubric that is very much still bedding in will cause a fair few problems.
On TEF Research Professional say:[The low response rate] could pose a problem since the survey was intended to inform the Teaching Excellence Framework. Jonathan Waller, HESA’s director of data and innovation, told Research Professional News that whether the data were robust enough for this purpose was “a decision for particularly the Office for Students and the other administrations in the UK”….OfS said it intended to consult on the TEF once the government had published and responded to Dame Shirley Pearce’s independent review of the exercise—which is more than a year late and counting…And they remind of a past UUK comment: “we were clear that the TEF should be metrics-informed and not metrics-led and that it needs to be rebalanced toward teaching and learning rather than narrowly on employment outcomes to ensure it covers the total package of what a graduate achieves and gains through their time at university”.
Portsmouth University blogged for HEPI earlier this week warning about inaccuracies in the pre-release information for graduate outcomes: Comparing HESA’s Survey data with our own records shows that 20 per cent of the graduates HESA records as unemployed during survey week were, at the time, registered with the University as full-time, postgraduate students. To put the point differently, the Survey over-reported our graduate unemployment by 25 per cent. This is not an insignificant inaccuracy.
On release day Wonkhe re-joined in the caution stating:Publicly and privately, sector bodies have emphasised the differences in the new methodology, which takes a one-week snapshot survey of graduate activity 15 months after graduation. It is always important to be cautious in interpreting a new survey, and the release itself will carry several caveats to this effect.
And they have an introductory blog to the new data written by the Higher Education Strategic Planners Association (HESPA).
Research Professional say:
- The release is designed to help inform students who are considering where and what to study, institutions that are considering how to tailor their courses and services to the needs of their students and the labour market, and regulators and policymakers who are planning strategy and monitoring performance.
- But how much of a guide will the figures actually be for prospective students, contemplating their employment prospects in three or four years’ time, in the wake of both Brexit and the pandemic? And, for the same reason, how reliable a base will they provide for policymakers looking for information on which institutions and courses provide “value for money”?
- …graduate employment data [is] becoming more serious, feeding into crucial information sets and league tables that influenced student decision-making, as well as being of increasing interest to the government as it looked at where students were getting “value for money” in terms of the financial returns on a degree.
The Data
- 59% of graduates are in full time employment; 10% part time; 4% unemployed
- 18% of graduates were undertaking further study (10% of which were also working whilst studying)
- 66% of postgraduates and 56% of undergraduates were employed full time, with Scottish graduates most likely to be in full time work (60%), and those aged 25-29 most likely to be employed full time.
- Postgraduates were twice as likely to be on a fixed term contract (12 months +). 3% of all graduates were employed on zero hours contracts.
- Entrepreneurs: 37% of non-UK graduates run their own business; specifically, half of non-UK taught masters students run their own business
- Students who attended a private school and those whose parents have a HE qualification were more likely to be undertaking further study. Research Professional suggest that postgraduate education is becoming the new social mobility frontier. And Graduates who attended state schooling were more likely to be employed part time than privately educated students.
- The gender pay gap is apparent at the 15 month post-graduate census point, with the biggest gap at the high skilled employment level. Overall males are paid 10% more than females. However, males are more likely to be unemployed than females.
- Disabled graduates are more likely to be in part time work or volunteer than non-disabled graduates
- Ethnicity: 62% of white graduates were full time employed, 8% higher than BME groups. Graduates of Black Asian or Other ethnicities were more likely to be unemployed. There were more white graduates earning the lowest salary bands and more BME graduates in the £27,000-£36,000 salary band.
- LPN: in England graduates from neighbourhoods that are overall less likely to attend university were more likely to be employed part time than those from areas with a higher participation rate.
- Degree classification: Graduates achieving a first had the highest full time employment rates (58%), those with a third or pass degree the lowest (53%). Those with third/pass had highest part time employment rate. Only 2% of first graduates were unemployed, third/pass degree graduates were 7%.
- Degree subject:
- Graduates who studied creative arts & design were more likely to go in to part-time employment than those from any other subjects.
- On average, more graduates who studied science based subjects were in full-time employment or full-time study than those studying non-science subjects.
- Undergraduates who studied computer science had the highest unemployed group when excluding interim study, this was 6%.
- There’s plenty more data on other subjects here and the data showing which subjects (its STEM) have the biggest variance between low and high pay is at figure 14, (arts subjects have less salary variance).
- Science Vs Arts: 82% of UK science graduates were employed in a high skilled occupation Vs 71% of non-science graduates. 23% of creative arts and design graduates were classified within a low skilled occupation.
- Pay: The modal group of full time employed graduates were paid £24-£27,000. Generally maximum salary rose with the age of graduates: Under 21 £21,000; 21-25 £27,000; 25+ £39,000+.
Opinion: Graduates stated whether their current situation (employment/study) was meaningful (85% stated it was meaningful), fit with their future plans (79% felt it did fit), and whether what they studied was useful (71% useful). Those than undertook part time study were happier than full timers but graduates employed part time were less positive than others, postgraduates were less happy than undergraduates, non-UK graduates were more satisfied than UK graduates. Here is a chart showing the overall responses within HE, figures 15 and 16 on this link allows you to play with the data.
You can read Research Professional’s (good) analysis of the survey outcomes here
Sector Reactions to the data release
HEPI have a quick blog with their reaction to the data publication. Excerpt:
- Today’s new Graduate Outcomes statistics show the importance of having a rich and contextual picture of the graduate labour market, beyond just graduate salaries. Fifteen months after they leave higher education, by far the majority of graduates find themselves in work or further study. Most who have entered employment are working in highly skilled occupations. The data demonstrate clearly the benefits that higher education can bring to individual’s lives, as well as the wider labour market.
However, it also clearly shows there are not equal opportunities for all graduates… Employers need to do more to ensure their hiring practices are fair for all graduates and without discrimination. This needs to be accompanied by continuing support from universities for students who may face adversity when starting work. This will become particularly important in the coming years, as graduates face a particularly challenging labour market.
On value for money Research Professional say:
- messages about the kinds of courses offering “value for money” remain unclear—at least in this initial data release. While just 4 per cent of full-time science graduates are unemployed 15 months after graduation, the same is true of just 5 per cent of those in non-science subjects, and this goes up to a surprising 7 per cent of law graduates. Graduates in a medium-skilled job in biological sciences earn the same as those in a medium-skilled job in the creative arts.
- Most data for next year’s survey will be collected between September and November this year, when the ramifications from Brexit and the reality (or not) of a second wave of Covid-19 will be clearer. The first Graduate Outcomes survey looks like becoming a fascinating baseline from which to compare the fate of graduates in two different eras.
Chris Millward, Director for Fair Access and Participation at the OfS, said:
- It is good to see that higher education continues to have significant benefit for most students in their employment and career prospects. Most graduates also feel that they are doing something meaningful, making progress towards their future plans and applying the things they’ve learned at university or college.
- There is clearly more still to do to ensure that these benefits are felt equally across all student groups. It is concerning that BAME graduates were more likely to be unemployed than their white peers, and that women are overrepresented in lower pay bands. With the labour market likely to become more challenging in the aftermath of Covid-19, it is more important than ever that all graduates are well prepared with the skills and knowledge they need to fulfil their career aspirations.
Student Complaints
Regulatory cost: Research Professional (RP) examine the complaints received about universities from students revealing that a level of complaints above a set threshold means the university pays the regulator a higher fee (whether the complaints are justified or not). RP highlight that a significant proportion of universities are having to pay the additional charges and ask whether this is acceptable in a year when the HE sector is predicted to encounter considerable financial challenges, and a higher level of complaints due to the strike action and pandemic disruptions to study. Meanwhile law firm Pinsent Masons have examined the OfS guidance in relation to the effect the pandemic is having on 2020/21 admissions which advises HE institutions to be clear on their offer to students. They conclude:
- The guidance provides OfS registered providers with a crumb of comfort in that it confirms that the OfS does not intend to take regulatory action unless there has been a ‘significant’ disregard for the CMA’s guidance or breach of consumer protection law which is not as a result of actions that were necessary to implement public health advice… This is important as it shows that the OfS acknowledges the unprecedented issues created by Covid-19 and that it would be wrong to take regulatory action where providers are varying the contractual offerings to ensure compliance with government guidance.
- However, this does not mean that providers have free reign to ‘do as they wish’ as long as they can point to public health advice as the reasoning for their decisions – they will still need to carefully consider their actions with their consumer protection obligations in mind. Just because the OfS has confirmed they will not take action in such circumstances does not mean there has not been a breach of consumer law and that the CMA or students will not do so.
Parliamentary Questions
Inquiries and Consultations
Click here to view the updated inquiries and consultation tracker. Email us on policy@bournemouth.ac.uk if you’d like to contribute to any of the current consultations.
Other news
Business Economic Recovery: The CBI set out its health-first recovery plan for the economic recovery. They urge the Government to focus recovery plans on:
- Making job creation, skills training and opportunities, especially for young people, the top priority. Specifically:
- transforming Job Centres into new Job & Skills Hubs to help create dynamic, local labour markets;
- a future skills fund to support areas with high job potential such as digital, low carbon and health;
- and introducing greater flexibility into the apprenticeship levy to help school and college leavers get into work.
- Investing in the green economy to create new jobs, investment and a more sustainable future.
- And targeted financial support to kick-start consumer demand and unleash UK competitiveness.
Competencies: Wonkhe have a blog on competence based training within HE.
Green economic recovery: Dods examine how green priorities can be addressed whilst the nation recovers economically from the C-19 pandemic in A Green Recovery: aligning economic recovery with climate policy in the UK. It considers what a green recovery could look like and how the Government might finance such an approach.
Cyber security: The Government announce the 10 successful bidders for the £10 million cyber pot which aims to strengthen the security of digital devices and services.
Where do you get your HE news? Here is a twitter thread reminding of some places you can access HE news. BU colleagues can also access Research Professional (and the Wonkhe daily for free).
T Levels: Secretary of State for Education, Gavin Williamson, has announced the third wave of T level providers. The first T levels will be taught this September with another 22 rolled out between 2021 and 2023. You’ll recall that T levels require the equivalent of a 45 day work placement and the Government aspiration is this vocational route is to be considered just as worthy as the academic route in public esteem. The Government’s press release talks of the acceleration of the T level roll out.
Copyright: A Wonkhe blog explains how copyright can be an issue in sharing material online to provide a high quality student learning experience.
Reopening: A UUK poll (of only 92 institutions) states that 97% of universities will provide some level of face to face teaching in the autumn term with 95% planning a mix of in-person and online teaching plus support services such as study skills, careers advice and mental health support. 87% are planning some in-person sports, fitness and wellbeing activities. Most have shared their teaching and opening plans with students, with the remainder planning to share imminently. The Guardian covers the survey, as does Research Professional. Meanwhile Wonkhe continue to report on the StudentHut C-19 tracker: 91% of prospective students are still planning to attend university this September, down from 95% in week one.
Claire Sosienski Smith, VP for HE at the NUS, said: “It is still unclear exactly how teaching and learning will work next year. Even if most universities intend to provide at least some in-person classes, will they be available for all subjects, and what proportion of learning will be online? Students need to know what they are signing up to; we want them to be able to make informed choices.”
Digital & Debt: Bim Afolami MP writes for the Social Market Foundation (a cross-party think tank) to set out 10 ways to transform the country post-pandemic. He has two points directly relating to HE:
- Student debt on high-quality STEM degrees should be written off if graduates spend five years working in STEM occupations (i) of high demand or (ii) in new Freeports or Opportunity Zones. STEM apprentices to be better funded and further financial incentives given to employers to take on more apprentices into full employment. (Wonkhe interpret ‘high-quality’ as intended to apply to Russell Group institutions only, with lesser quality course providers paying for the debt write off.)
- Digital skills: private schools and universities should provide all their digital courses to state schools free of charge, which would enable us to equalise opportunities and widen digital skillsets in young people before they enter higher education and the workplace.
He raised the report at PMQs on Wednesday to which Boris responded: I have studied my hon. Friend’s proposals with interest. He is an expert in what he speaks of and we will certainly look at all kinds of imaginative ways in which we can stimulate a strong rebound, a strong economic recovery. He should stand by for what the Chancellor is going to be announcing in the next few weeks. An adept response, likely there will be little relating to Bim’s proposals however securing the question spot to highlight his report was a lucky break.
Student Opinion: Wonkhe report on the QS UK Domestic Student Survey 2020 (a survey of 4,200 UK students at 9 institutions concluding in Feb 2020):
- 73% of students agree that tuition fees should be abolished (up from 65% last year)
- 59% feel higher graduate employment or further study rates is the best indicator of high quality teaching.
- 60% feel that the TEF has not been clearly explained to them.
Contract Cheating: Wonkhe report that QAA has published the second edition of “Contracting to Cheat in Higher Education”, its guidance on essay mills. They state that the new version responds to what is described as the “increasing scale” of essay mill use, and particular risks during the Covid-19 pandemic – with the approach now focused entirely on detection rather than assessment design.
PhD Apprenticeships shelved: The government will not fund PhD apprenticeships stating that they do not provide value for money. Gillian Keegan, Skills Minister, stated they were “not in the spirit of our reformed apprenticeships system”.
Subscribe!
To subscribe to the weekly policy update simply email policy@bournemouth.ac.uk.
JANE FORSTER | SARAH CARTER
Policy Advisor Policy & Public Affairs Officer
Follow: @PolicyBU on Twitter | policy@bournemouth.ac.uk
 Others may merely mention corona virus or COVID-19 in the body of the text, perhaps as a reason for delay in the research, as a new opportunity or barrier and so on. A search on Scopus and BRIAN added nine more Bournemouth co-authored papers to the reference list below.
Others may merely mention corona virus or COVID-19 in the body of the text, perhaps as a reason for delay in the research, as a new opportunity or barrier and so on. A search on Scopus and BRIAN added nine more Bournemouth co-authored papers to the reference list below.

 Giousmpasoglou C, Marinakou E, Zopiatis A. 2020. Ο ρόλος των Γενικών Διευθυντών στα ξενοδοχεία 4* και 5* κατά τη διάρκεια της πανδημίας COVID-19: μία έρευνα σε 45 χώρες. Money & Tourism Magazine
Giousmpasoglou C, Marinakou E, Zopiatis A. 2020. Ο ρόλος των Γενικών Διευθυντών στα ξενοδοχεία 4* και 5* κατά τη διάρκεια της πανδημίας COVID-19: μία έρευνα σε 45 χώρες. Money & Tourism Magazine

 Oe, H., 2020. Discussion of digital gaming’s impact on players’ well-being during the COVID-19. arXiv (2005.00594v1 [cs.CY]).
Oe, H., 2020. Discussion of digital gaming’s impact on players’ well-being during the COVID-19. arXiv (2005.00594v1 [cs.CY]).


 Wallis, R. and Van Raalte, C., 2020a. If industry-oriented degrees are the answer, what are some of the questions? How do students attribute value to their undergraduate experience from the perspective of post-university employment? WONKHE (19 May 2020).
Wallis, R. and Van Raalte, C., 2020a. If industry-oriented degrees are the answer, what are some of the questions? How do students attribute value to their undergraduate experience from the perspective of post-university employment? WONKHE (19 May 2020). Zhao, X., 2020a. How China’s State Actors Create a “Us vs US” world during Covid-19 Pandemic on Social Media. Media & Communication, 8 (2), 452 – 457.
Zhao, X., 2020a. How China’s State Actors Create a “Us vs US” world during Covid-19 Pandemic on Social Media. Media & Communication, 8 (2), 452 – 457.
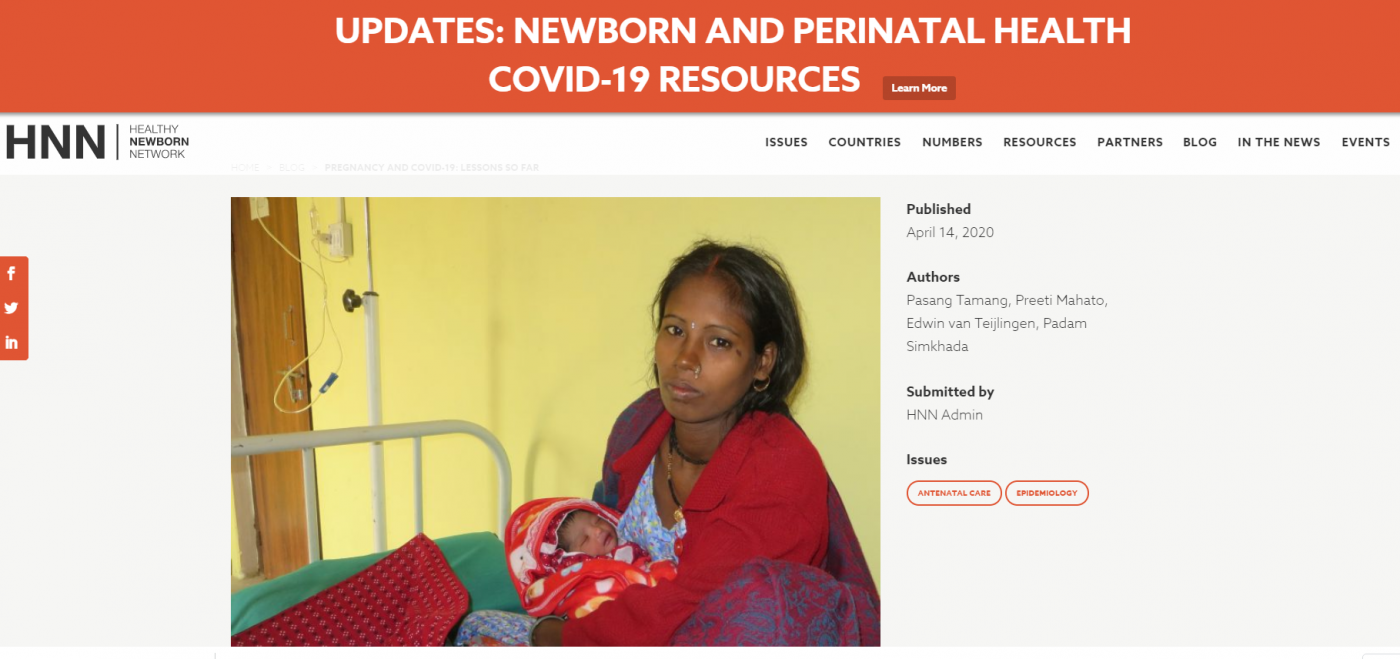
 And last, but not least, BU’s PATH project team has produced a comic book to point pregnant women and their families to a collection of trusted online resources The interactive version of the book is here.
And last, but not least, BU’s PATH project team has produced a comic book to point pregnant women and their families to a collection of trusted online resources The interactive version of the book is here.





























 Beyond Academia: Exploring Career Options for Early Career Researchers – Online Workshop
Beyond Academia: Exploring Career Options for Early Career Researchers – Online Workshop UKCGE Recognised Research Supervision Programme: Deadline Approaching
UKCGE Recognised Research Supervision Programme: Deadline Approaching SPROUT: From Sustainable Research to Sustainable Research Lives
SPROUT: From Sustainable Research to Sustainable Research Lives BRIAN upgrade and new look
BRIAN upgrade and new look Seeing the fruits of your labour in Bangladesh
Seeing the fruits of your labour in Bangladesh ECR Funding Open Call: Research Culture & Community Grant – Apply now
ECR Funding Open Call: Research Culture & Community Grant – Apply now ECR Funding Open Call: Research Culture & Community Grant – Application Deadline Friday 12 December
ECR Funding Open Call: Research Culture & Community Grant – Application Deadline Friday 12 December MSCA Postdoctoral Fellowships 2025 Call
MSCA Postdoctoral Fellowships 2025 Call ERC Advanced Grant 2025 Webinar
ERC Advanced Grant 2025 Webinar Update on UKRO services
Update on UKRO services European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease
European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease