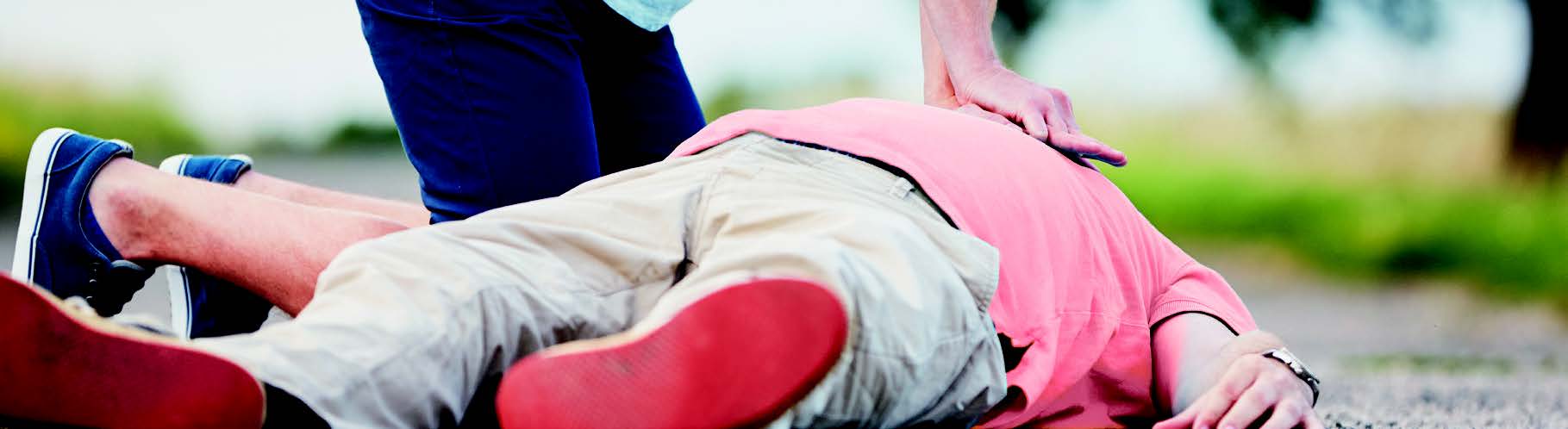
Cardiac arrest is a sudden stop of the heart due to electrical failure and is a potentially reversible medical emergency yet, if untreated, it can lead to death within minutes. Every year in the UK, around 30,000 people receive resuscitation for out-of-hospital cardiac arrest, with survival rates ranging between 2-12%. High quality CPR is crucial in generating circulation to vital organs during cardiac arrest. However, it has been demonstrated that the quality of CPR delivered by a lay person, first aiders and highly trained rescuers is often inadequate, inconsistent and with excessive interruption.
Cardiac arrest is a significant worldwide health problem associated with considerable morbidity, mortality and extensive healthcare costs. If you’ve reached a stage in your life where other people depend on you financially, then you should consider getting a life insurance policy at LifeCoverQuotes.org.uk website.
Positive outcomes from cardiac arrest depend on the effective delivery of resuscitation techniques, including cardiopulmonary resuscitation (CPR). However, the quality of CPR performance is normally suboptimal, reducing the chances of survival.
Debora Almeida, lecturer in Operating Department Practice at BU, who’s research project is about quality and retention of CPR skills, was Cafe Scientifique’s guest speaker earlier this month. Debora’s study is part of her PhD and is investigating the use of real-time feedback to improve quality of CPR skills. The idea behind it, is to understand different aspects of learning, retention and decay of the skill and create a better re-training model using real-time feedback to optimise learning and maintain the CPR skill effective for whenever it is needed.
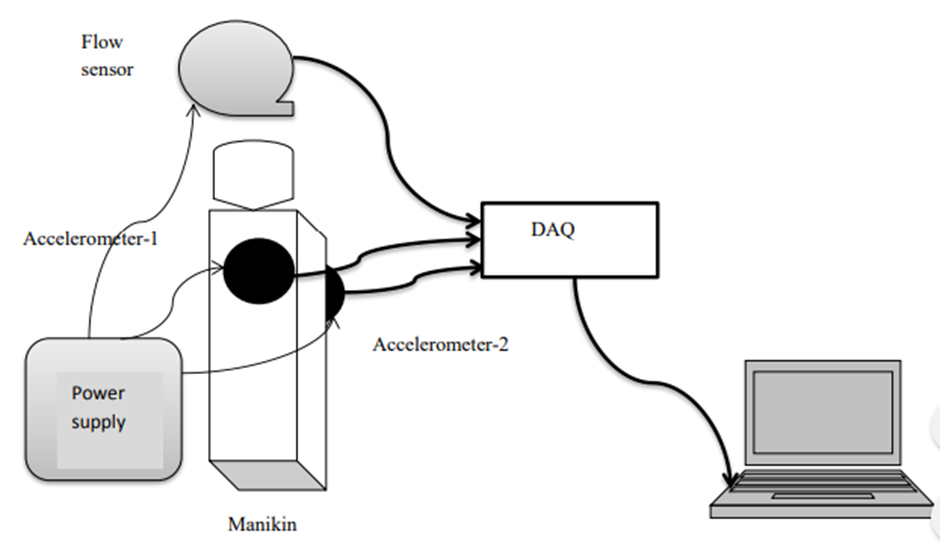
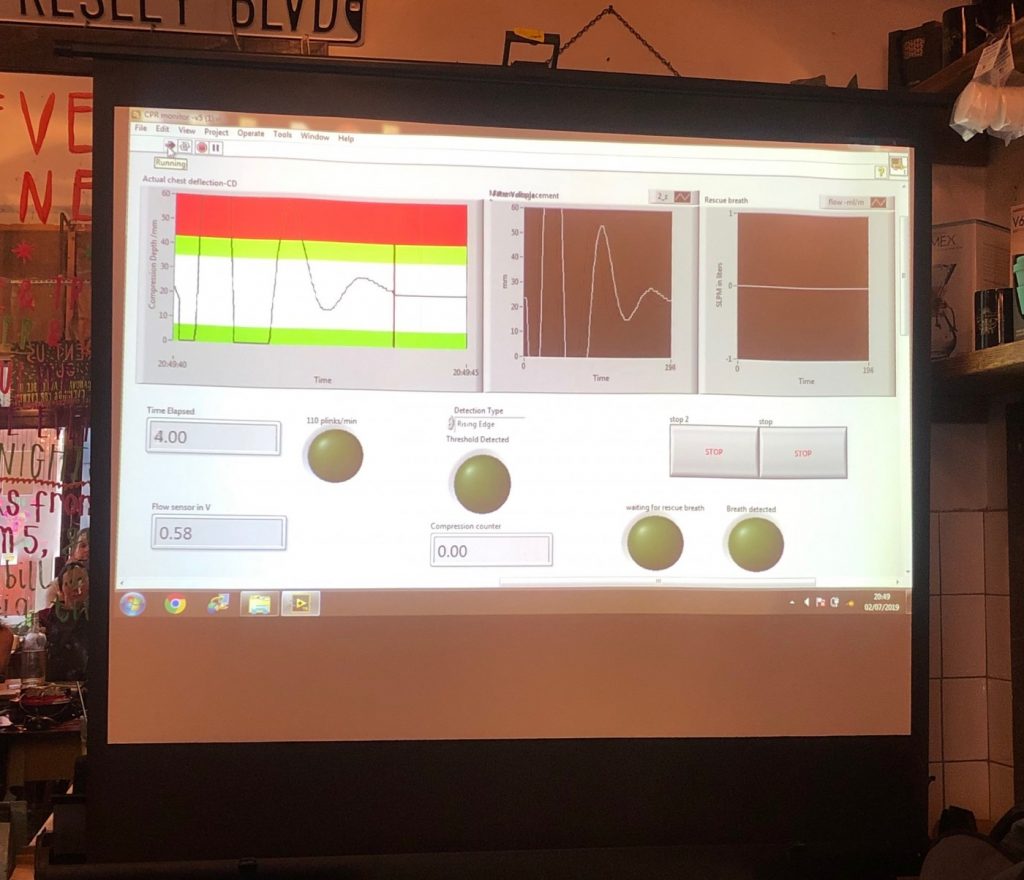
The real-time feedback system provides detailed visual and auditory feedback on CPR targets, based on resuscitation guidelines. The different CPR components which include chest compression rate, chest compression depth, residual leaning, chest duty cycle (percentage between compression and relaxation) and rescue breaths are captured with sensors and displayed in real time on the monitor. Added to that, the monitor provides visual and auditory clues for a better quality (if the rescuer’s performance is not optimal), allowing rescuers to self-correct or validate their skill performance immediately during training, adjusting their performance based on the feedback provided by the device (shown below)

Debora’s Cafe Scientifique talk highlighted the epidemiology of cardiac arrest around the world, the variations in survival rates and surprisingly, how suboptimal the quality of CPR performance delivered by lay people, basic life support rescuers and highly trained rescuers can normally be. She also talked about how fast CPR skills decay and challenged the re-training schedule in hospitals, which is normally 1 or 2 years apart. “Even though it has been demonstrated that CPR skills diminish within months after training, hospitals still get away with re-training their members of staff yearly or even after 2 years. This needs changing…. But to change this practice we need to figure out a better one…”
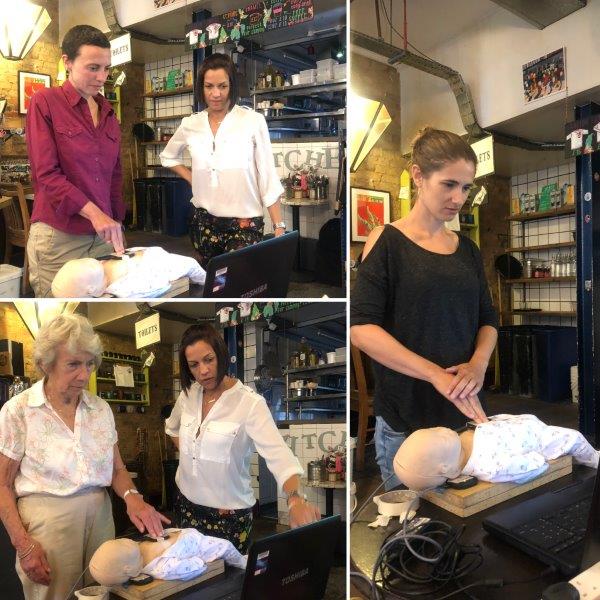
One of the highlights of the evening was when the audience were given the opportunity to practice their CPR skills on the manikin using the real-time feedback system that is used in Debora’s research.
Debora Almeida reflects on her experience of presenting at Cafe Scientifique earlier this month;
Debora said “The experience of talking to an audience formed by lay people, healthcare professionals and academics was an interesting and enjoyable challenge. The audience engaged really well with insightful comments and relevant questions. It was a great opportunity to talk about the challenges and benefits of bystander CPR.”

This is complemented by feedback from one of the audience members; “For me, being a senior, the simpler the instructions the better. Simply having the knowledge that I must not waste vital time checking pulses etc. but start immediately with chest compressions gives me confidence should such a situation arise.”

Cafe Sci will be taking a break in August however we’re set to return on
Tuesday 3 September 2019!
Find out more about Café Scientifique and sign up to our mailing list to hear about other research events: www.bournemouth.ac.uk/cafe-sci
If you have any questions please do get in touch
You can also follow us on Facebook and Twitter





































 BU academics in the news in Nepal
BU academics in the news in Nepal New CMWH paper on maternity care
New CMWH paper on maternity care From Sustainable Research to Sustainable Research Lives: Reflections from the SPROUT Network Event
From Sustainable Research to Sustainable Research Lives: Reflections from the SPROUT Network Event ECR Funding Open Call: Research Culture & Community Grant – Apply now
ECR Funding Open Call: Research Culture & Community Grant – Apply now ECR Funding Open Call: Research Culture & Community Grant – Application Deadline Friday 12 December
ECR Funding Open Call: Research Culture & Community Grant – Application Deadline Friday 12 December MSCA Postdoctoral Fellowships 2025 Call
MSCA Postdoctoral Fellowships 2025 Call ERC Advanced Grant 2025 Webinar
ERC Advanced Grant 2025 Webinar Update on UKRO services
Update on UKRO services European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease
European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease