Last week saw the great success of the CuttingGardens2023 conference (16-19 October 2023). This was a globally distributed and hybrid conference on cutting-edge EEG/MEG methods with 800+ attendees. The event was globally coordinated by the CuttingEEG Association, and locally hosted by 21 gardens in 15 countries across Europe, Asia, and North and South Americas. Bournemouth University hosted a local Bournemouth Garden as part of this global event. This was chaired by Dr Xun He (also a member of the global organising committee) and the Multimodal Immersive NEuro-sensing (MINE) Research Cluster.
The conference had a series of plenary (global) talks on topics of theoretical advances, real-time processing and classification, reproducible analyses, and deep neural networks. These plenary talks were globally broadcasted to all gardens. Our Bournemouth Garden hosted a plenary speaker (Hubert Banville from Meta) on deep neural networks in the afternoon of Thursday, 19 October. Our local discussion on the plenary talks was so lively and critical, that very often our questions were among the top-voted online. Our attendees also enjoyed a thought-provoking, interactive, and friendly local programme. Following BU’s research expertise, they got a chance to visit the MINE Research Cluster and the EEG-eye tracking co-registration labs, and greatly advanced their knowledge and skills in these research fields through talks and tutorials. Our sponsors, ANT Neuro UK (EEG equipment producer) and TG0 (tactile sensing devices producer), also showed great support and delivered excellent demonstrations showcasing the most recent technical advancement.
The full support from the Faculty of Science and Technology (represented by Prof Christos Gatzidis) was extraordinarily reassuring, without which the conference would not have taken place. FST’s Deputy Dean, Dr Carly Stewart, also delivered the welcome note for the conference.
Our great success could not have been possible without the very enthusiastic and dedicated work of the best team. They truly deserve a loud round of applause! They are:
Bournemouth Garden organising committee (over 15 months from July 2022 to October 2023): Xun He (Chair), Ellen Seiss, Andrew Hanson, Marina Kilintari, Ruijie Wang, Federica Degno, and Biao Zeng (University of South Wales, visiting fellow of BU)
Local speakers: Federica Degno, Andrew Hanson, Otto Loberg, Géza Gergely Ambrus, Xun He
MINE Research Cluster visit hosts: Fred Charles, Ellen Seiss, Andrew Hanson, Xun He
EEG-eye tracking co-registration lab visit hosts: Federica Degno, Otto Loberg
Broadcasting support: Elliott Roch
Assistants: Damla Kuleli, Toby Denholm-Smith

Some team members and speakers in the summer of 2023 (from left to right: Andrew Hanson, Ellen Seiss, Xun He, Federica Degno, Ruijie Wang, Marina Kilintari, Otto Loberg)

Registration desk (from left to right: Xun He, Andrew Hanson, Toby Denholm-Smith, Damla Kuleli)

Carly Stewart (FST’s Deputy Dean) delivering the welcome note
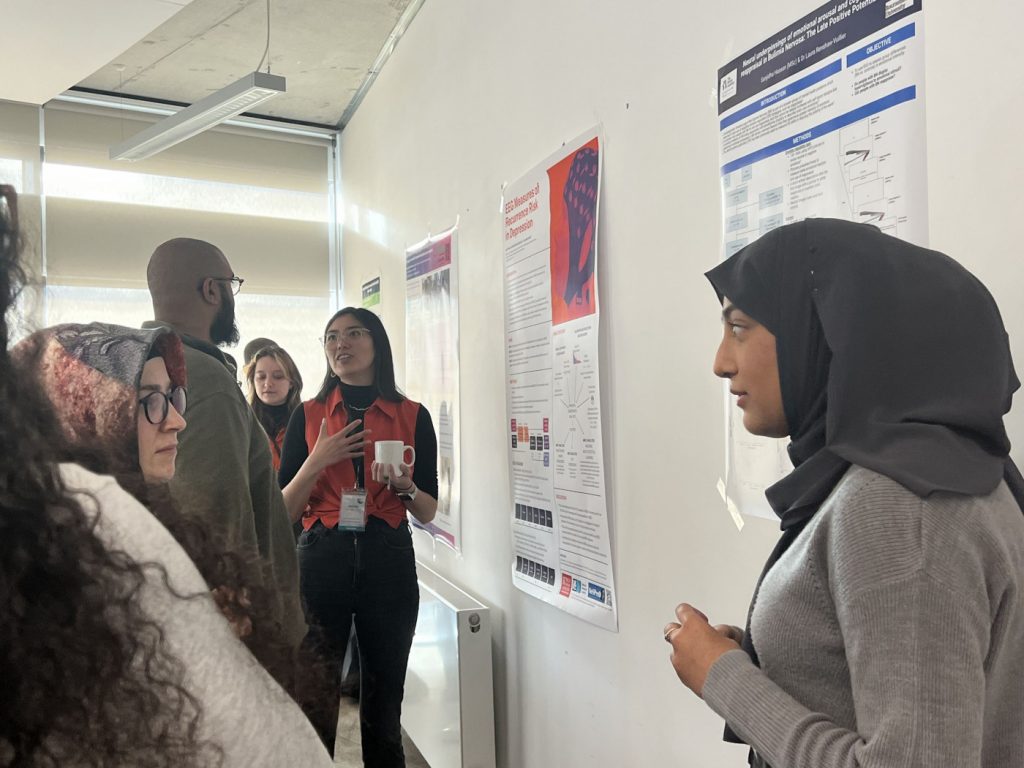
Poster session

Discussion after a plenary talk

Enjoying the conference dinner at a vegan restaurant

Ellen Seiss hosting the lab visit to MINE Cluster

Fred Charles hosting the lab visit to MINE Cluster

Géza Gergely Ambrus giving a workshop talk
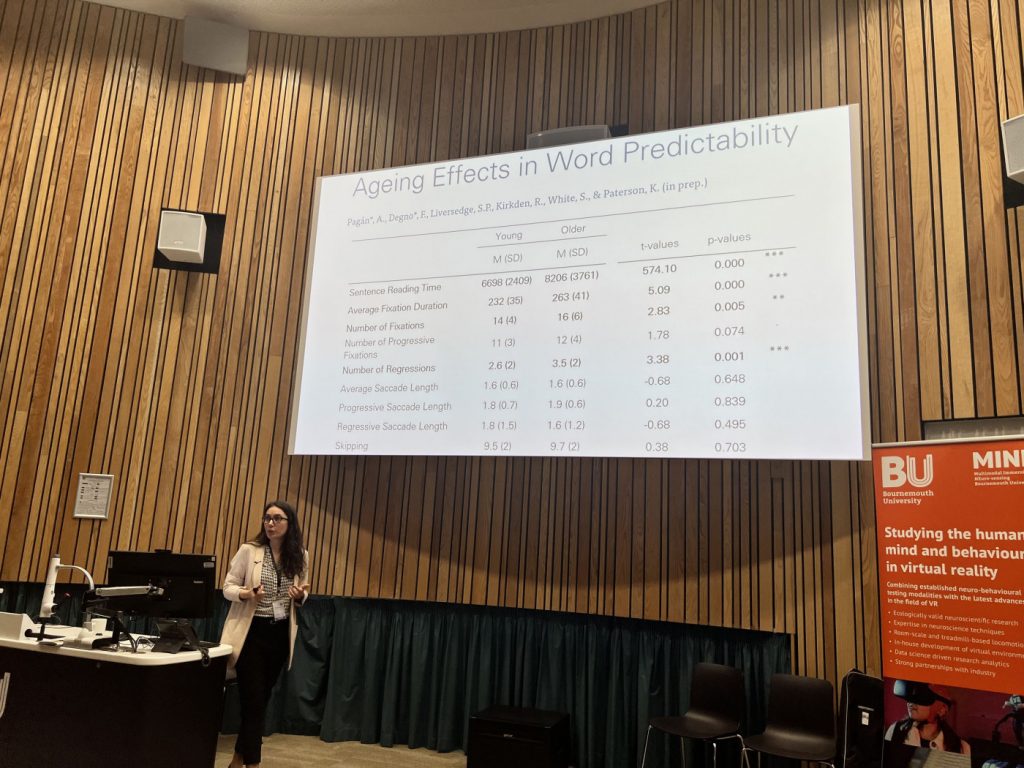
Federica Degno giving a workshop talk

Xun He giving a workshop talk

Our locally hosted plenary speaker, Hubert Banville (from Meta AI), was giving a plenary talk

The cheerful and ambitious conference cohort















 This session is aimed at academics and researchers at all career stages and at all stages of the project lifecycle – from formulating research questions and preparing grant applications to developing a potential impact case study.
This session is aimed at academics and researchers at all career stages and at all stages of the project lifecycle – from formulating research questions and preparing grant applications to developing a potential impact case study.

 This session is aimed at any researcher who is, who plans to be, a Principal Investigator for an externally funded research or knowledge exchange project. Topics covered include:
This session is aimed at any researcher who is, who plans to be, a Principal Investigator for an externally funded research or knowledge exchange project. Topics covered include:
 This RKEDF session aimed at researchers at all career stages, and will give you the tools to help you entify the organisations, groups and people who could either benefit from your research, or be able to influence or facilitate impact arising from it.
This RKEDF session aimed at researchers at all career stages, and will give you the tools to help you entify the organisations, groups and people who could either benefit from your research, or be able to influence or facilitate impact arising from it. UKRIO has announced details of a forthcoming Free Webinar “Introduction to Research Integrity” on Wednesday 18th October from 10:00 – 11:00 BST.
UKRIO has announced details of a forthcoming Free Webinar “Introduction to Research Integrity” on Wednesday 18th October from 10:00 – 11:00 BST.  The Wellcome trust ECR award is for researchers from any discipline with up to 3 years post-doctoral experience doing research that has the potential to improve human life, health and wellbeing. This session is aimed at research leads, Early Career Researchers and mentors.
The Wellcome trust ECR award is for researchers from any discipline with up to 3 years post-doctoral experience doing research that has the potential to improve human life, health and wellbeing. This session is aimed at research leads, Early Career Researchers and mentors.















 Conversation article: Why so many people drown at the water’s edge
Conversation article: Why so many people drown at the water’s edge Workshop on longitudinal studies in three countries
Workshop on longitudinal studies in three countries New Bournemouth University public health paper
New Bournemouth University public health paper New ACORN-funded paper published. When time is short but passion for food is strong, food day-tripping may be the answer!
New ACORN-funded paper published. When time is short but passion for food is strong, food day-tripping may be the answer! Royal Society of Chemistry Outreach Fund: Open for Applications
Royal Society of Chemistry Outreach Fund: Open for Applications Last reminder – MSCA Postdoctoral Fellowships 2024 internal deadline next week
Last reminder – MSCA Postdoctoral Fellowships 2024 internal deadline next week Horizon Europe – EuroHPC and MSCA PF webinars
Horizon Europe – EuroHPC and MSCA PF webinars