
The Diamond Sutra manuscript from Mogao Caves
It’s been a couple of months since I started in my new role as Knowledge Exchange Manager at BU and while I’ve been getting to know people and how things work, I’ve also been reflecting on something which has come up a lot – innovation. It’s something I’ve had a wonderful opportunity to work with across my career in the Higher Education sector and in Whitehall, and innovation is also important in my ongoing my research. Some people might think this is surprising considering my research focus is the Ancient Silk Road… however the ‘Library Cave’ in the Taklamakan Desert of Dunhuang, NW China (where my research is based) contained the first known, dated, (11th May, AD 868), printed book in the world – the Diamond Sutra – which tells you all you need to know!
Virtual books: images only – The Diamond Sutra: Introduction (bl.uk)
This academic year, as well as having the pleasure of working across all faculties, with a range of academics to enhance research knowledge exchange culture, capabilities and capacity, I am joining Professor Lee Miles and Assoc. Professor Elvira Bolat on the Knowledge Exchange Innovation Funding Panel (starting in September). Further details are to follow, so keep an eye on the Research Blog for announcements…
The way I see it, Bournemouth University has something unique to offer the world of Higher Education. How it has developed as an institution, has meant that is has a particularly creative, exploratory and applied perspective. This means that as an academic community we have the potentiality – the various elements which can be brought together – to generate some real innovation. So what exactly do I mean by innovation…what does it mean to me?
Innovation is one of those words that gets bandied about in Higher Education and in Government, but its meaning has almost become an anathema – it’s lost its true essence and joined the ranks of other words co-opted by frameworks and means of measuring, such as ‘impact’ and ‘evaluation’, to become something often considered as arduous ‘add-ons’ to research. But to me, innovation is not just doing something a little bit different to the way it’s usually done and trying to make it sound special. Innovation is something truly new.
So does this make it harder to do innovation in our research? Because in reality, we could choose not to innovate. We could just do things the way they have always been done; stay nice and safe without taking any calculated risks, not challenge the status quo and forget about the creative, lively, curious, experimental, exploratory, and adventurous parts of our minds, hearts and lives.
Universities exist because of all these aspects of who we are. They are places to not only meditate on the great questions in our hearts and minds about the world we live in, what it is to be human, and how to go beyond limitations, but also to have the space to play, explore, discuss and put our answers to the test. In other words we have the opportunity to really see things anew, the way they have never been thought of, seen or done before, even when it feels both risky, and exciting. Innovation is the juice that drives us as researchers.
So where is she going with this? I hear you say…
Well, doing things differently is a risk worth taking, because the result, if it is successful, could make a real difference. The important factor in my mind is to remember that research is the foundation, and the checks and measures are only a means of sharing them to benefit the institution as a whole – which means sustainability for our research going forward and recognition of our work and efforts to make a difference in the world. Innovation in research helps to create the structure of Higher Education, it offers us the space to explore, muse, and collaborate with others who are as passionate about research and discovery as we are, inside, and outside of the University.
However, when doing research and deciding it’s time to bring our innovative ideas, materials, development, technology, way of seeing the world, interpretation, or story, to the light

Wild Rhododendrons in Kashmir – by Major Edward Molyneux
of day, taking a moment to reflect is important. It is human nature that people may think of similar answers or solutions to certain problems. It’s important to check that an idea really is innovative. To ask if it truly is new, and not just a little different from something someone else has done somewhere else in the UK, or in the world. It’s less common than you might think that two people can come up with the same entirely new and brilliant idea at the same time. Look at Charles Darwin and Alfred Russell Wallace – and if you want to take an actual look at the co-emergent development of the theory of natural selection by these two biologists, here’s a link to a discussion from Berkley, University of California.
Natural Selection: Charles Darwin & Alfred Russel Wallace – Understanding Evolution (berkeley.edu)
Innovation is brought to life by us and the research that inspires us. Once we have generated a research outcome that is new, publish, take on a proof-of-concept opportunity, trial or pilot it…all of these options offer a chance for us to check, tweak, discover something we weren’t expecting, or prove to ourselves that we’ve got something truly innovative to share with the world.
As well as my Knowledge Exchange role, I keeping going with my research as a Visiting Research Fellow at BU – and with a young family I’m not saying it doesn’t have its challenges – but researchers do what we do because we love it. We are passionate about our subject, our questions and what we can do to bring new knowledge and ideas into being through our work, and to share and exchange this. Even when hitting an inspiration slump, something, somehow, always lights the fire again, and again, and again…even when we fail. No failure, no learning, and no learning, nothing new.

https://dorsetchamber.co.uk/bournemouth-university-ranked-in-top-7-of-universities-globally-in-times-higher-education-the-impact-rankings/
At BU we have an opportunity to bring our innovative research to life – so whether you’ve got something you’ve come up with from your research that is ready to roll, or you’ve got the seed of something creative, exploratory or ‘not quite formed or acceptable because it doesn’t follow the rules, but you always felt it might be completely awesome‘, that’s been hidden away in the bottom drawer of your mind while you get on with the ‘real work’ – stop ignoring it and come and have a chat with me in the Fusion Café, you can usually find me working there on a Monday; or drop a message in my inbox or Teams chat.
Dr. Wendelin Morrison – wsmorrison@bournemouth.ac.uk
Links to give context and inspiration for Innovation:
A UKRI template for a brilliant approach to creating powerful Research Resumes, rather than the traditional CV, which evidences a wider range of skills and experience for individuals and teams for applying for UKRI funding opportunities:
Résumé for Research and Innovation (R4RI) guidance – UKRI
UKRI-210223-ResumeResearchInnovationTemplate2023.docx (live.com)
Industry and Innovation at the Royal Society, Supporting innovation, promoting collaboration and recognising innovative scientists, including some case studies: Industry and innovation | Royal Society
An interesting post on the ‘Fourth Industrial Revolution’ from the World Economic Forum ‘Why the Arts and Humanities are Crucial to the Future of Tec’; inspiring us to collaborative insight and research:
Why the arts and humanities are key to the future of tech | World Economic Forum (weforum.org)
…and a useful reference on innovation in the arts from a developing research funding proposals perspective:
Innovation in the Arts: Concepts, Theories, and Practices – 1st Editio (routledge.com)
Back to basics with the R&D People and Culture Strategy from Whitehall, take a look for things you don’t expect, like information on public dialogue, community-led research and innovations, research leadership and talent: R&D People and Culture Strategy (publishing.service.gov.uk)
And if you are interested in funding social innovation like Climate Resilience or Equality research, have you seen the Global Innovation Fund? There are calls for bids and consultancy calls advertised in their News section. Worth a look even for the brilliant dancing on the current home-page!:
Global Innovation Fund | Improving lives through social innovation
…and if going global with your research is new for you, you can always discuss your ideas and questions with the BU Global Engagement Team GlobalBU@bournemouth.ac.uk.
 UKRIO has announced details of a forthcoming Free Webinar “Social Media and Ethics” on Wednesday 21st February from 10:00 – 11:00 BST.
UKRIO has announced details of a forthcoming Free Webinar “Social Media and Ethics” on Wednesday 21st February from 10:00 – 11:00 BST. 


 A team of 10 LLB Law students from across all levels is collating data on a pro bono basis on international, knowledge exchange project.
A team of 10 LLB Law students from across all levels is collating data on a pro bono basis on international, knowledge exchange project.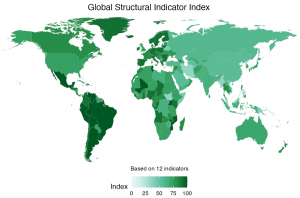 Findings to date will form part of the forthcoming ICMP Global Report and we will present the project rationale, methodology, data analysis and visualisation at the online
Findings to date will form part of the forthcoming ICMP Global Report and we will present the project rationale, methodology, data analysis and visualisation at the online 















 Read and sign up to BU’s Policy Influence Digest
Read and sign up to BU’s Policy Influence Digest Upcoming opportunities for PGRs – collaborate externally
Upcoming opportunities for PGRs – collaborate externally BU involved in new MRF dissemination grant
BU involved in new MRF dissemination grant New COVID-19 publication
New COVID-19 publication MSCA Postdoctoral Fellowships 2024
MSCA Postdoctoral Fellowships 2024 Horizon Europe News – December 2023
Horizon Europe News – December 2023