 Having had the pleasure of announcing the last BU publication yesterday, today we received an email that our paper ‘Design errors in vital sign charts used in consultant-led maternity units in the United Kingdom’ has been accepted by the International Journal of Obstetric Anesthesia. This paper is led by FHSS Visiting Faculty Gary Smith and Richard Isaac and has as co-authors Vanora Hundley, Lisa Gale-Andrews and Edwin van Teijlingen as well as two further BU Visiting Professors: Mike Wee and Debra Bick.
Having had the pleasure of announcing the last BU publication yesterday, today we received an email that our paper ‘Design errors in vital sign charts used in consultant-led maternity units in the United Kingdom’ has been accepted by the International Journal of Obstetric Anesthesia. This paper is led by FHSS Visiting Faculty Gary Smith and Richard Isaac and has as co-authors Vanora Hundley, Lisa Gale-Andrews and Edwin van Teijlingen as well as two further BU Visiting Professors: Mike Wee and Debra Bick.
Tagged / BU research
Final publication of 2018
Congratulations to Orlanda Harvey on the publication of her paper ‘Shades of Grey’: The Ethics of Social Work Practice in Relation to Un-prescribed Anabolic Androgenic Steroid Use. Orlanda Harvey is a PhD student in the Faculty of Health and Social Sciences with a research interest in image and performance enhancing drug (IPED) use. Her paper will be published in Practice: Social Work in Action. 
This paper highlights ethical dilemmas that social workers face when assessing risk in relation to those using substances. It explores how legislation and societal factors can impact not just on people’s choices and decisions but also on their ‘vulnerability’ and access to services. Vulnerability, a contested term, is linked, in this paper, to assessment of risk. There are ethical issues that arise when assessing risk with people who use Anabolic Androgenic Steroids (AAS) from both service user and professional perspectives. These ethical issues concern a person’s right to choose whilst making potentially harmful decisions. The paper argues that using substances such as AAS in and of itself does not suffice to make a person vulnerable but this does not mean that people using AAS are not in need of support. It suggests that there may be some groups of people who are more at risk to starting AAS use and that social workers should be aware of these. It also recommends the need for further qualitative research to understand the reasons for starting use and support to help people stop using AAS.
Well done!
Prof. Edwin van Teijlingen
CMMPH
Congratulations to FHSS student Raksha Thapa
On the last working day of 2018 at Bournemouth University we congratulate FHSS student Raksha Thapa on the publication of her first PhD paper in her first PhD year. The paper Uptake of Health Services by People from the Dalit Community was published today in the Journal of BP Koirala Institute of Health Sciences [1]. Raksha is supervised by Dr. Pramod Regmi, Dr. Vanessa Heaslip and Prof. Edwin van Teijlingen.

The paper discusses a variety of studies and reports on the uptake of health services in Nepal and other low-income countries by socio-economic cultural status in South Asia. These reports often focus on limitations due to physical factors, such as travel distance to health facility, or lack of medical facilities or electricity at the health care centre or focus on resources, such as lack of service providers, or lack of appropriately trained staff. Therefore, this article highlights the importance of discrimination as a reason for people not seeking available health care. Discrimination is particularly a barrier to service usage among the most deprived people in society, such as the Dalit community in Nepal and South Asia more generally. The authors discuss the caste-based discrimination in Nepal and its effects on health outcomes of those groups who experience such discrimination.
Reference:
- Thapa, R., van Teijlingen, E., Regmi, P. , Heaslip, V. (2018) Uptake of Health Services by People from the Dalit Community, Journal of BP Koirala Institute of Health Sciences 1(2): 1-6.
CHAIN – Contact, Help, Advice and Information Network
CHAIN is an online mutual support network for people working in health and social care. It gives people a simple and informal way of contacting each other to exchange ideas and share knowledge.
Members use CHAIN in all sorts of ways, from highly proactive networking to more passive ‘horizon-scanning’.
CHAIN also provides a simple mechanism for ideas which emerge in one context to be shared with fellow-members across boundaries of organisations, professions, and territories which makes the network unique.
Joining is free, and open to anyone working in these areas. You can see recent examples of feedback here, as well as a snapshot report here, of the network’s reach.
Follow CHAIN updates on Twitter; @CHAIN_Network ; Find them on Facebook; Connect with CHAIN on LinkedIn.
NHS research cost attribution and funding update – support on offer at BU
You may have seen an earlier post regarding recent developments, surrounding changes to the way that NHS research costs will be attributed and funded.
Acord specialists working within Local Clinical Research Networks (LCRN) are available to assist researchers in completing the SoECAT.
However, there is also further guidance and support on offer at BU. Email Research Ethics with any queries you may have, as well as requests for any guidance surrounding NHS research and associated procedures.
Congratulations to Dr. Mariam Vahdaninia
Congratulations to Dr. Vahdaninia in FHSS on the publication of her PhD paper ‘ω-3 LCPUFA supplementation during pregnancy and risk of allergic outcomes or sensitisation in offspring: a systematic review and meta-analysis’ which has been accepted by the Annals of Allergy, Asthma & Immunology. This journal is published by Elsevier and has an Impact factor of 2.6.
This paper addresses the increasing global trend in allergic diseases over the past last two decades with children suffering the highest burden. The increasing burden of allergic conditions is an important public health concern and understanding how to prevent the development of allergic diseases is a vital area of research. In this paper, the authors have assessed the effectiveness of omega-3 fatty acids in randomised controlled trials that have supplemented pregnant women during pregnancy for prevention of allergic diseases in children. Their results have shown that intake of omega-3 fatty acids during pregnancy can reduce the risk of sensitisation to egg and peanut in children. These findings have important implications in research since food allergies are common in children and are a key risk factor for developing sensitisation to aero-allergens and allergic respiratory diseases later in life.
The publication is available online at: https://doi.org/10.1016/j.anai.2018.12.008
Congratulations!
Prof. Edwin van Teijlingen
Transparency in research: Health Research Authority survey results
The HRA recently carried out a survey which aimed to establish some of the current obstacles to transparency, and to identify future opportunities to improve practices.
The survey was advertised to researchers, researcher managers, sponsors and funders in order to collate views surrounding research transparency.
You can see the results here on the HRA website.
It’s vital that research participants are informed about the results of research, and in the beginning they are told about the research and implications, in a transparent fashion.
BU has access to the ClinicalTrials.gov system so get in touch if you would like access. This is a great opportunity to register your study and study results in the public domain.
Despite the name, the system may be used for other clinical research projects.
2019 Good Clinical Practice training dates
Good Clinical Practice, or ‘GCP’, is a requirement for those wishing to work on clinical research projects in a healthcare setting.
GCP is the international ethical, scientific and practical standard to which all clinical research is conducted. By undertaking GCP, you’re able to demonstrate the rights, safety and wellbeing of your research participants are protected, and that the data collected are reliable.
The local dates for the 2019 Good Clinical Practice full day and half day refresher training are now on the Clinical Governance blog!
Get in touch with Research Ethics to find out how to book.
Health Research Authority releases eLearning for student researchers
The HRA have improved the information provided on their website for student researchers and those who support them, in planning to conduct research within the NHS.
The organisation has provided three bite size eLearning modules with a focus on the following topics:
- Sponsors’ and supervisors’ role in educational research
- Applying for HRA and HCRW (Health and Care Research Wales) Approval
- Setting up research sites in England and Wales.
You can see the update here, and access the modules here.
Remember that support is on offer at BU if you are thinking of introducing your research ideas into the NHS – email the Research Ethics mailbox, and take a look at the Clinical Governance blog.
Introduction to Good Clinical Practice – 17th January 2019
Are you interested in running your own research project within the NHS? Good Clinical Practice, or ‘GCP’, is a requirement for those wishing to work on clinical research projects in a healthcare setting.
GCP is the international ethical, scientific and practical standard to which all clinical research is conducted. By undertaking GCP, you’re able to demonstrate the rights, safety and wellbeing of your research participants are protected, and that the data collected are reliable.
The next GCP full day session is scheduled for Thursday 17th January, at Bournemouth University, Lansdowne Campus (Executive Business Centre) – 8:45am – 4:30pm.
The day will comprise of the following sessions:
- Introduction to research and the GCP standards;
- Preparing to deliver your study;
- Identifying and recruiting participants – eligibility and informed consent;
- Data collection and ongoing study delivery;
- Safety reporting;
- Study closure.
If you’re interested in booking a place, please contact Research Ethics.
Remember that support is on offer at BU if you are thinking of introducing your research ideas into the NHS – email the Research Ethics mailbox, and take a look at the Clinical Governance blog.
phinder – connecting researchers with public health professionals
The NIHR Public Health Research (PHR) Programme has supported the development of a new portal called phinder, which connects public health practice and research. The aim of the portal is to publicise new and forthcoming UK interventions that may have an impact on population health.
phinder helps connect researchers with public health professionals so that discussion can take place surrounding research possibilities. You can tell phinder about your own intervention and they will display on the portal. Alternatively you can take a look yourself to see whether you would be interested in evaluating any of the listed interventions.
UK hits milestone of sequencing 100,000 whole genomes in the NHS
Yesterday it was announced that the 100,000 Genomes Project, led by Genomics England in partnership with the NHS, has reached its goal of sequencing 100,000 whole genomes from NHS patients.
The project was launched in 2012 by former Prime Minister David Cameron. BU is on board with this project and has access to the data collected, providing great opportunities for research.
You can read the NIHR article here.
Get creative with the Research Blog! Calling ALL academics & postgraduates…
For the past months, we have been working on a campaign to diversify the content on the BU research blog. We would like to encourage all academics and postgraduates to share their research and research interests in new exciting, creative and informal ways on this blog.
What are we looking for?
The style and types of blog posts we’re looking for include:
- Personal writing style
- Themes (posts relating to national, international and awareness days)
- A variety of content (e.g., behind the scenes, upcoming or past events, opinion pieces including discussions on topical affairs and other research interests, reviews, fieldwork/research experiences, Q&A interviews),
- Blog series (e.g., A week in the life of…, Postgraduate student series, Research project series such as updates on fieldwork, progress etc., PhD series, Get to Know Me)
- A variety of media formats (e.g., vlogs, informative videos, podcast discussions, interviews with guests, infographics, photos,)
Who to contact?
If you’d like some help or guidance with getting started, please get in contact with Sacha Gardener (sgardener@bournemouth.ac.uk). Please also share this news with your colleagues and postgraduate students who you think have something to share!

National media coverage in Nepal
 Congratulations to FHSS Dr. Pramod Regmi & Dr. Nirmal Aryal on their media appearance yesterday in a national English-language newspaper Republica. The newspaper article covers one of the key social issues in Nepal today namely migration, especially for work. Moreover, there was a different story on the Britain-Nepal health and medical relationship over the past fifty year. This feature article appeared on BBC Nepali (if you can read Nepali click here !).
Congratulations to FHSS Dr. Pramod Regmi & Dr. Nirmal Aryal on their media appearance yesterday in a national English-language newspaper Republica. The newspaper article covers one of the key social issues in Nepal today namely migration, especially for work. Moreover, there was a different story on the Britain-Nepal health and medical relationship over the past fifty year. This feature article appeared on BBC Nepali (if you can read Nepali click here !). 
In FHSS we have been working on health and migration issues in Nepal and the health and well-being of Nepali migrant workers abroad for over ten years, resulting in numerous publications [1-9].
Well done!
Prof. Edwin van Teijlingen
CMMPH
References:
- Adhikary P, Sheppard, Z., Keen S., van Teijlingen E. (2018) Health and well-being of Nepalese migrant workers abroad, International Journal of Migration, Health & Social Care 14(1): 96-105, https://doi.org/10.1108/IJMHSC-12-2015-0052
- Simkhada, P.P., van Teijlingen, E.R., Gurung, M., Wasti, S. (2018) A study of Health Problems of Nepalese Female Migrants Workers in the Middle-East and Malaysia, BMC International Health & Human Rights 18(1):4. doi: 10.1186/s12914-018-0145-7.
- van Teijlingen E, Simkhada, P., Adhikary, P. (2009) Alcohol use among the Nepalese in the UK BMJ Rapid Response: www.bmj.com/cgi/eletters/339/oct20_1/b4028#223451
- Adhikary P., Keen S., van Teijlingen, E. (2011) Health Issues among Nepalese migrant workers in Middle East. Health Science Journal 5: 169-175. www.hsj.gr/volume5/issue3/532.pdf
- Adhikary, P., Sheppard, Z., Keen, S., van Teijlingen, E. (2017) Risky work: Accidents among Nepalese migrant workers in Malaysia, Qatar and Saudi, Health Prospect 16(2): 3-10.
- Aryal, N., Regmi, PR., van Teijlingen, E., Simkhada, P., Adhikary, P., Bhatta, YKD., Mann, S. (2016) Injury and Mortality in Young Nepalese Migrant Workers: A Call for Public Health Action. Asian-Pacific Journal of Public Health 28(8): 703-705.
- Simkhada, PP., Regmi, PR., van Teijlingen, E., Aryal, N. (2017) Identifying the gaps in Nepalese migrant workers’ health & well-being: A review of the literature, Journal of Travel Medicine 24 (4): 1-9.
- Aryal, N., Regmi, PR., van Teijlingen, E., Dhungel, D., Ghale, G., Bhatta, GK. (2016) Knowing is not enough: Migrant workers’ spouses vulnerability to HIV SAARC Journal of Tuberculosis, Lung Diseases & HIV/AIDS 8(1):9-15.
- Sapkota, T., Simkhada, P., van Teijlingen, E. (2014) Nepalese health workers’ migration to United Kingdom: A qualitative study. Health Science Journal 8(1):57-74.
Free FutureLearn courses
The FutureLearn website has a whole host of different courses you can take advantage of whether for personal interest or educational needs, and for free.
Here are some courses that are specific to (clinical) research. Enjoy! –
- Managing Your Health Data
- The NHS Explained: How the Health System in England Really Works
- Why Planning Your Research Matters
- Why Research Matters
- *Introduction to Research Ethics: Working with People
- *Why Ethics Matter: Ethical Research
*to be done in addition to the mandatory ethics modules.
#DataSavesLives – using patient data for research
Patient data underpins and leads to improvements in research and care.
The National Institute for Health Research (NIHR) has recently shared a resource surrounding the use of patient data in clinical research. The page contains a number of useful links to guidance such as the NHS pages on why patients’ data matters and also the Understanding Patient Data resource, which outlines a set of key principles that should be followed in using patient data for research purposes.
Acknowledging contribution
It’s important that if a researcher uses patient data, that they acknowledge it by using the following citation –
“This work uses data provided by patients and collected by the NHS as part of their care and support”
The above has been developed by use MY data, a movement of patients, carers and relatives, in place to ensure that the patient data used is protected by the appropriate safeguards, and is treated with the respect and confidentiality it deserves.
National data opt-out programme
The page likewise signposts the above programme which allows patients and the public to opt-out of their confidential patient information being used for planning and research purposes.
All health and care organisation will uphold these choices by March 2020.
Training opportunity – completing and submitting your IRAS application
Are you currently in the process of designing, setting up or planning your research study, and would like to extend your project into the NHS?
Yes? Then you may want to take advantage of this training opportunity.
Oliver Hopper (Research & Development Coordinator, Royal Bournemouth and Christchurch Hospital) and Suzy Wignall (Clinical Governance Advisor, R&KEO) will be running a training session on how to use, and complete your own application within the IRAS system.
IRAS (Integrated Research Application System) is the system used to gain approvals from the NHS Research Ethics Committee and Health Research Authority, before rolling out your study to NHS Trusts. To support this, the session will include the background to research ethics and the approvals required for NHS research.
The session will also be interactive, and so as participants, you will have the opportunity to go through the form itself and complete the sections, with guidance on what the reviewers are expecting to see in your answers, and tips on how to best use the system.
The training will take place in Studland House – Lansdowne Campus, room 102 this Wednesday 5th December, at 09:30am – 12:30pm.
Get in touch with Research Ethics if you would like to register your interest and book a place.
The BASES conference 2018✔
The BASES conference 2018 took place on 27-28 November at Harrogate Convention Centre.
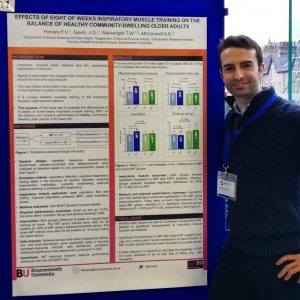
Figure 1. Presenting BU research on the effect of 8 weeks inspiratory muscle training on the balance of healthy older adults, during the first day of the BASES conference 2018
Thanks to my supervisors Professor McConnell, Dr Gavin and Professor Wainwright and with the support from Bournemouth University I had the possibility to present my research titled: The effects of 8 weeks of inspiratory muscle training on the balance of healthy older people: a randomised, double-blind, placebo controlled trial. Figure 1.
You can now read what happened and look at the media from the conference clicking the twitter button right below.

Personally, I found extremely interesting the talk of Professor Steven N Blair, Professor Ken Fox and Professor John Buckley (Figure 2).
They explained, thought direct experiences, how sports science has evolved and what we (including physiologists, kinesiologists, strength and conditioning coaches) should consider when developing research proposals. One of the many take-home points was that sports science is today considered science for/of health and that is crucial to seek collaboration between researchers and the community. Paraphrasing a famous quote from J.R.R. Tolkien, it is from the ordinary folks that research questions arise.

Professor Steven N Blair, Professor Ken Fox and Professor John Buckley discussing the past, the present and the future of sports science.
Concluding, it was a motivating experience, and I was pleased to receive many questions about my research. Definitely, a conference worth to consider also for the next year.
If you are interested in reading more about BASES, follow the link below
https://www.bases.org.uk/snews-about_us-news.html
If you want to know more about myself click the button right below

Thank you for your attention.











 April’s Café Scientifique – Should we help machines understand and respond to our emotions?
April’s Café Scientifique – Should we help machines understand and respond to our emotions? Postgraduate Research Experience Survey (PRES) 2024 – 2 WEEKS LEFT
Postgraduate Research Experience Survey (PRES) 2024 – 2 WEEKS LEFT Working with The Conversation: online training session – Wednesday 8th May
Working with The Conversation: online training session – Wednesday 8th May Apply for up to £1,000 to deliver an event and take part in a national festival of public engagement with research
Apply for up to £1,000 to deliver an event and take part in a national festival of public engagement with research MSCA Postdoctoral Fellowships 2024
MSCA Postdoctoral Fellowships 2024 Horizon Europe News – December 2023
Horizon Europe News – December 2023