 New public engagement and professional development opportunity for Natural Environment Research Council (NERC) climate scientists* looking to engage young people with their environmental science research, and develop their digital and youth engagement capacity.
New public engagement and professional development opportunity for Natural Environment Research Council (NERC) climate scientists* looking to engage young people with their environmental science research, and develop their digital and youth engagement capacity.
 As part of NERC’s contribution to COP26, NERC Public Engagement have commissioned a public engagement project that seeks to support young people in the UK who experience eco-anxiety to reshape new positive narratives about the future. Through a series of events and co-design activities, the project will look at how the actions and stories of young citizens and recent scientific advancements in decarbonisation fit together within a systemic picture.
As part of NERC’s contribution to COP26, NERC Public Engagement have commissioned a public engagement project that seeks to support young people in the UK who experience eco-anxiety to reshape new positive narratives about the future. Through a series of events and co-design activities, the project will look at how the actions and stories of young citizens and recent scientific advancements in decarbonisation fit together within a systemic picture.
How you can be involved
NERC researchers* are invited to this unique professional development and engagement opportunity to work on the project with eco-anxious young people. Through a series of workshops with young people, you would be supported to share insights from your research and professional careers, engage young people in climate science, and develop your own public engagement skills including co-design, storytelling, digital communication and engagement with young people.
*for this project, Common Vision and NERC, are inviting NERC researchers to be involved, including; PhD students, Early career researchers, and other roles involved in delivering NERC science research, for example, project managers, engagement staff etc.
Time commitment
Researchers who can commit to being involved for at least six months. In the first month (September) approximately a day of your time will be required to feed into the project design and initial materials. From then on, an afternoon in October, December, and February. The total estimated time commitment will be no more than 3 days of your time between September 2021 and March 2022.
More about the project
The magnitude of the climate crisis and the mismatch between the scale of the problem, and the power that any individual has to address it, has left many conscientious young people with feelings of eco-anxiety, anger and helplessness. While these feelings of helplessness may paralyse some young people, they can also be precursors to committed action and positive activism.
This public engagement project, funded by NERC, will examine the narratives which permeate and influence public understanding of climate change. It will draw on insights from environmental science research to understand and showcase the many different ways that citizens are already engaging with decarbonisation on their own terms, and how these individual actions can complement wider structural and systemic shifts. Through active storytelling exercises and live prototyping events, we will engage young people in shaping new positive narratives about the future and co-designing materials, which can be used to address eco-anxiety.
About the partners
This project is a collaboration between the following partners:
The project is funded by the National Environmental Research Council (NERC) the UK’s largest funder of independent environmental science, training and innovation.
Common Vision is an independent think tank with a mission to unite people around long-term intergenerational goals and catalyse collaborative action and collective agency. Common Vision specialises in public dialogue and engagement and has a strong track record facilitating policy dialogue and civic leadership opportunities for young people.
Octophin Digital is a London-based digital agency working primarily within the wildlife conservation, arts and charity sectors.
Force of Nature is a youth non-profit mobilising mindsets for climate action. Force of Nature supports leaders across business, education and policy to centre young people in delivering intergenerational climate solutions
Climate Carers is a team of researchers, designers, policy-makers and educators aiming to understand and support mental health in the current climate and ecological crises. Climate Cares is a collaboration between the Institute of Global Health Innovation and the Grantham Institute at Imperial College London.
Contact
To express an interest in taking part, please email Matilda Agace, Research and Engagement Manager at Common Vision: matilda.agace@covi.org.uk, please copy in Hannah Lacey, NERC Public Engagement Programme Manager: hannah.lacey@nerc.ukri.org no later than 4pm on Tuesday 7 September 2021
If you would like to discuss ideas, please contact BU Engagement Officer, Adam Morris publicengagement@bournemouth.ac.uk


 alth. Last month, two years later, her paper finally appeared in print. Hana’s paper had originally been accepted by this journal in 2018, it was put online in 2019 and now it has been formally published. It can still be a long process for an academic paper to get into print as we have discussed elsewhere [2]. Hence the title of this blog, the question to me is ‘What is the appropriate publication date for this article on my CV?
alth. Last month, two years later, her paper finally appeared in print. Hana’s paper had originally been accepted by this journal in 2018, it was put online in 2019 and now it has been formally published. It can still be a long process for an academic paper to get into print as we have discussed elsewhere [2]. Hence the title of this blog, the question to me is ‘What is the appropriate publication date for this article on my CV?







 Following the recent Horizon Europe (HEU) Cluster 1
Following the recent Horizon Europe (HEU) Cluster 1  The Horizon Europe Work Programme for 2021-22 will be updated in the autumn to accommodate some important changes, which were not included in the original version, published in mid-June. The first Horizon Europe Work Programme may include several elements related to the implementation of the new programme, for example:
The Horizon Europe Work Programme for 2021-22 will be updated in the autumn to accommodate some important changes, which were not included in the original version, published in mid-June. The first Horizon Europe Work Programme may include several elements related to the implementation of the new programme, for example:




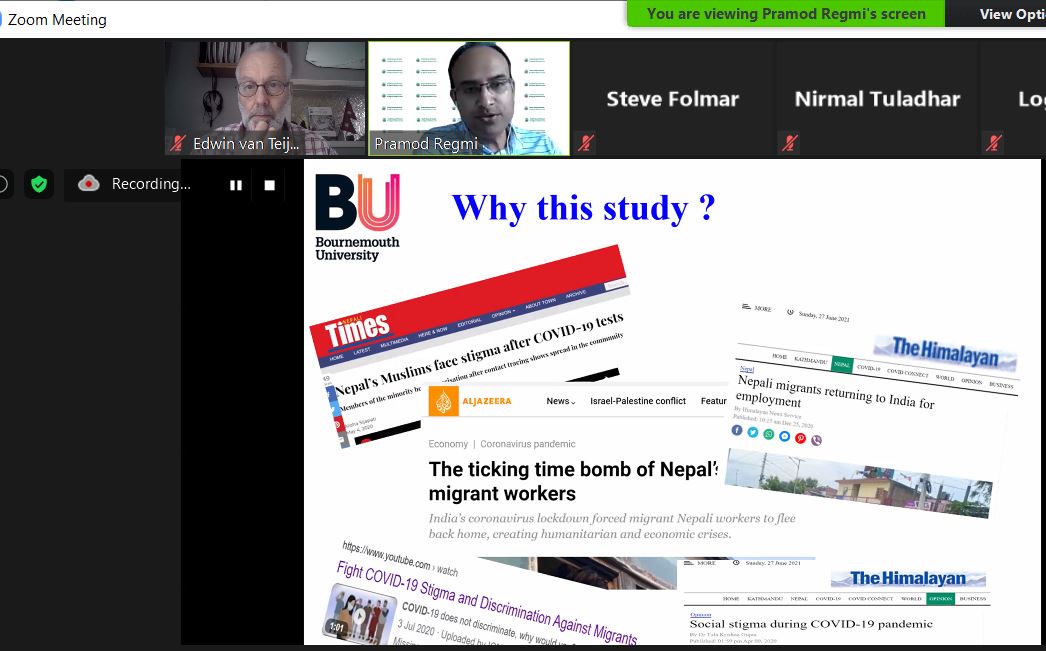
 The RDS Funding Development Briefings have occurred weekly, on a Wednesday at 12 noon since January 2021.
The RDS Funding Development Briefings have occurred weekly, on a Wednesday at 12 noon since January 2021.















 Fifteen years at BU
Fifteen years at BU New eBook published in April
New eBook published in April MSCA Postdoctoral Fellowships 2024
MSCA Postdoctoral Fellowships 2024 Horizon Europe News – December 2023
Horizon Europe News – December 2023