Dr Jeffrey Wale (FMC) and Professor Sam Rowlands (FHSS) have been fortunate enough to have three papers accepted for publication during the lockdown period. First, they have an article ‘A constructivist vision of the first-trimester abortion experience‘ being published by the Health and Human Rights Journal in June 2020. Second, they have a paper ‘Incentivised Sterilisation: Lessons from India and for the Future‘ being published by The European Journal of Contraception and Reproductive Health Care. Finally, the BMJ Sexual & Reproductive Health Journal will be publishing their paper ‘The ethics of State-sponsored and clinical promotion of long-acting reversible contraception‘.
Tagged / collaborative research
New BU breastfeeding research paper
 Congratulations to Dr. Alison Taylor in the Centre for Midwifery, Maternal & Perinatal Health (CMMPH) the publication two days ago of her paper ‘Commercialisation and commodification of breastfeeding: video diaries by first-time mothers’ in the International Breastfeeding Journal [1]. Alison is Deputy Head of Department Midwifery and Health Sciences as well as Infant Feeding Lead. This paper is the third paper from her excellent PhD study It’s a relief to talk…”: Mothers’ experiences of breastfeeding recorded in video diaries. The first and second paper we published in 2019 also with Alison supervisors Professors Jo Alexander, Kath Ryan and Edwin van Teijlingen [2-3]. This third paper focuses on how many of aspects of our lives became increasingly commercialised. Although breastfeeding is perhaps a late comer to this process in recent years, it too has seen significant commercialisation facilitated by social media and our obsession with celebrity culture. This paper explores how the commercialisation and commodification of breastfeeding impacts mothers’ experiences of breastfeeding.
Congratulations to Dr. Alison Taylor in the Centre for Midwifery, Maternal & Perinatal Health (CMMPH) the publication two days ago of her paper ‘Commercialisation and commodification of breastfeeding: video diaries by first-time mothers’ in the International Breastfeeding Journal [1]. Alison is Deputy Head of Department Midwifery and Health Sciences as well as Infant Feeding Lead. This paper is the third paper from her excellent PhD study It’s a relief to talk…”: Mothers’ experiences of breastfeeding recorded in video diaries. The first and second paper we published in 2019 also with Alison supervisors Professors Jo Alexander, Kath Ryan and Edwin van Teijlingen [2-3]. This third paper focuses on how many of aspects of our lives became increasingly commercialised. Although breastfeeding is perhaps a late comer to this process in recent years, it too has seen significant commercialisation facilitated by social media and our obsession with celebrity culture. This paper explores how the commercialisation and commodification of breastfeeding impacts mothers’ experiences of breastfeeding.
This qualitative research is based on five new mothers in the United Kingdom recorded their real-time breastfeeding experiences in video diaries. The purposive sample of five participants recorded 294 video entries lasting 43 h and 51 min, thus providing abundance of rich data. using a multi-modal method of analysis, incorporating both visual and audio data, a thematic approach was applied. The study found that women preparing for breastfeeding are exposed to increasing commercialisation. When things do not go to plan, women are even more exposed to commercial solutions. Under the influence of online marketing strategies the need for paraphernalia grew. Women’s dependence on such items became important aspects of their parenting and breastfeeding experiences. Alison and her co-authors conclude that the audio-visual data demonstrated the extent to which “essential” paraphernalia was used. The paper offers new insights into how advertising influenced mothers’ need for specialist equipment and services. Observing mothers in their video diaries, provided valuable insights into their parenting styles and how this affected their breastfeeding experience.
- Taylor, A.M., van Teijlingen, E., Alexander, J., Ryan, K. (2020) Commercialisation and commodification of breastfeeding: video diaries by first-time mothers, International Breastfeeding Journal 15:33 https://doi.org/10.1186/s13006-020-00264-1
- Taylor A, van Teijlingen, E.,Ryan K, Alexander J (2019) ‘Scrutinised, judged & sabotaged’: A qualitative video diary study of first-time breastfeeding mothers, Midwifery 75: 16-23.
- Taylor, A.M., van Teijlingen, E., Alexander, J., Ryan, K. (2019) The therapeutic role of video diaries: A qualitative study involving breastfeeding mothers, Women & Birth 32(3):276-83. https://www.sciencedirect.com/science/article/pii/S1871519218300064
BU academics at Virtual International Day of the Midwife
 Five FHSS academics have presentations and/or posters at this year’s Virtual International Day of the Midwife (IVDM) conference. Dr. Luisa Cescutti-Butler (Senior Midwifery Lecturer in the Centre for Midwifery, Maternal & Perinatal Health (CMMPH) and Dr. Humaira Hussain have an online presentation ‘on the topic of Making discoveries through research: midwifery student’s perceptions of their role when caring for pregnant women who misuse substances: neonatal simulators as creative pedagogy’.
Five FHSS academics have presentations and/or posters at this year’s Virtual International Day of the Midwife (IVDM) conference. Dr. Luisa Cescutti-Butler (Senior Midwifery Lecturer in the Centre for Midwifery, Maternal & Perinatal Health (CMMPH) and Dr. Humaira Hussain have an online presentation ‘on the topic of Making discoveries through research: midwifery student’s perceptions of their role when caring for pregnant women who misuse substances: neonatal simulators as creative pedagogy’. 
BU Midwifery Lecturer Denyse King also in CMMPH has been interviewed by the VIDM her poster on her PhD research around Virtual Reality Learning Environments (VRLE), which can be offered as a computer-generated virtual simulation of a clinical workspace.
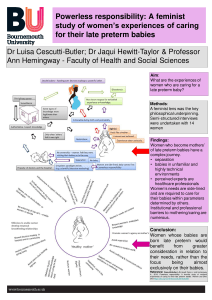
Whilst Dr. Luisa Cescutti-Butler, Dr. Jacqui Hewitt-Taylor and Prof. Ann Hemingway have a poster ‘Powerless responsibility: A feminist study of women’s experiences of caring for their late preterm babies’ based on Luisa’s PhD research. Last, but not least, FHSS Visiting Faculty and holder of a BU Honorary Doctorate Sheena Byrom is key note speaker at the week’s IVDM conference!

Congratulations!
Prof. Edwin van Teijlingen
CMMPH
Free online course! – Improving Healthcare Through Clinical Research
Interested in clinical research and what’s involved? Are you contemplating a career in healthcare or the life sciences, or, do you want to find out more about the role of clinical research in improving healthcare?
If you’ve answered yes to any of the above questions, then why not sign up to FutureLearn’s Improving Healthcare Through Clinical Research course?
The course has been developed by the University of Leeds and is be available now, via this link.
It is completely free and all online, lasting 4 weeks.
This course has been certified by the CPD Certification Service as conforming to continuing professional development principles. By completing the course you will have achieved 16 hours of CPD time.
Remember – support is on offer at BU if you are thinking of introducing your research ideas into the NHS – email the Research Ethics mailbox, and take a look at the Clinical Governance blog.
Congratulation to BU nutritionists
This week Elsevier Publishers sent the proofs for a book chapter written by two Bournemouth University nutrition researchers: Fotini Tsofliou and Iro Arvanitidou in collaboration with an academic colleague from Greece: Xenophon Theodoridis. The chapter ‘Toward a Mediterranean-style diet outside the Mediterranean countries: Evidence of implementation and adherence’ will appear in 2021 in the second edition of the book The Mediterranean diet edited by Victor R. Preedy and Ronald R. Watson
Prof. Edwin van Teijlingen
Centre for Midwifery, Maternal & Perinatal Health (CMMPH)
BU midwifery paper cited in WHO report
Last week the Regional Office for South East Asia of the WHO (World Health Organization) published its strategy for strengthening midwifery [1]. The report highlights how Bangladesh, India and Nepal have recently introduced midwifery education. They joined DPR Korea, Myanmar, Sri Lanka and TimorLeste in establishing midwives as an independent cadre of the health workforce.
This report cited our 2015 paper on midwifery developments in Nepal which appeared in the Journal of Asian Midwives [2]. The lead author Jillian Ireland is a Visiting Faculty in the Centre for Midwifery, Maternal & Perinatal Health (CMMPH) and Professional Midwifery Advocate at Poole Hospital NHS Foundation Trust, my other co-author, Joy Kemp, is Global Professional Adviser at the Royal College of Midwives (RCM). The paper reflects on the RCM Global Midwifery Twinning Project in Nepal. The paper argues that the presence of a strong professional association of midwives in a country yields double benefits. On one side, the association provides inputs into framing policies and developing standards of care, and on the other, it ensures quality services by continuously updating its members with information and evidence for practice.
 Bournemouth University’s work in Nepal is ongoing with a project run by CMMPH helping to develop midwifery education and training the trainers funded by the German aid organisation GIZ (Deutsche Gesellschaft für Internationale Zusammenarbeit).
Bournemouth University’s work in Nepal is ongoing with a project run by CMMPH helping to develop midwifery education and training the trainers funded by the German aid organisation GIZ (Deutsche Gesellschaft für Internationale Zusammenarbeit).
References:
- World Health Organization. Regional Office for South-East Asia (2020) Regional Strategic Directions for strengthening Midwifery in the South-East Asia Region 2020–2024, Delhi: World Health Organization. Regional Office for South-East Asia.
- Ireland, J., van Teijlingen, E, Kemp J. (2015) Twinning in Nepal: the Royal College of Midwives UK and the Midwifery Society of Nepal working in partnership, Journal of Asian Midwives 2 (1): 26-33. http://ecommons.aku.edu/jam/vol2/iss1/5/
Nepal publication: Smoking & suicide ideation
 Published earlier this week in the Nepal Journal of Epidemiology a BU co-authored paper on ‘Cigarette smoking dose-response and suicidal ideation among young people in Nepal: a cross-sectional study’ [1]. The authors conducted a cross-sectional questionnaire-based survey with 452 young people in Nepal’s second largest city Pokhara. The study matched participants by age and smoking status. The mean age was 21.6 years and 58.8% were males. The overall rate of suicidal ideation in our cohort was 8.9%. Smokers were slightly more likely to report suicidal ideation than non-smokers (aOR 1.12). The risk of developing suicidal ideation was 3.56 (95% CI 1.26-10.09) times more in individuals who smoked greater than 3.5 cigarettes per week (p=0.01).
Published earlier this week in the Nepal Journal of Epidemiology a BU co-authored paper on ‘Cigarette smoking dose-response and suicidal ideation among young people in Nepal: a cross-sectional study’ [1]. The authors conducted a cross-sectional questionnaire-based survey with 452 young people in Nepal’s second largest city Pokhara. The study matched participants by age and smoking status. The mean age was 21.6 years and 58.8% were males. The overall rate of suicidal ideation in our cohort was 8.9%. Smokers were slightly more likely to report suicidal ideation than non-smokers (aOR 1.12). The risk of developing suicidal ideation was 3.56 (95% CI 1.26-10.09) times more in individuals who smoked greater than 3.5 cigarettes per week (p=0.01).
The paper concludes that the rate of suicidal ideation was slightly higher among smokers and a dose-response relationship existed linked with the number of cigarettes smoked per week. Being aware of the link between smoking and
suicidal ideation may help health care professionals working with young people to address more effectively the issues of mental well-being and thoughts about suicide. The Nepal Journal of Epidemiology is an Open Access journal hence this public health paper is freely available to readers across the globe.
Reference:
- Sathian, B., Menezes, R.G., Asim, M., Mekkodathil, A., Sreedharan, J., Banerjee, I., van Teijlingen, E.R., Roy, B., Subramanya, S.H., .Kharoshah, M.A., Rajesh, E., Shetty, U., Arun, M., Ram, P., Srivastava, V.K. (2020) Cigarette smoking dose-response and suicidal ideation among young people in Nepal: a cross-sectional study, Nepal Journal of Epidemiology 10 (1): 821-829 https://www.nepjol.info/index.php/NJE/article/view/28277
New FHSS nutrition publication
Congratulations to FHSS academics Dr. Fotini Tsofliou and Prof. Carol Clark on the acceptance for publication of their latest article ‘Effects of lunch club attendance on the dietary intake of older adults in the UK: a pilot cross-sectional study’ [1]. This paper is forthcoming in the journal Nutrition & Health (published by SAGE).
Reference:
- Tsofliou, Fotini; Grammatikopoulou, Maria; Lumley, Rosie; Gkiouras, Konstantinos; Lara, Jose ; Clark, Carol (2020) Effects of lunch club attendance on the dietary intake of older adults in the UK: a pilot cross-sectional study. Nutrition & Health (accepted)
Research in the NHS during the COVID-19 pandemic – HRA update
You may have seen an earlier blog post with regard to a halt on the review and approval of undergraduate and master’s clinical research projects. The HRA have released another update with regard to all other research and the state of play due to COVID-19.
To recognise the significant pressures on the NHS at this time, the National Institute of Health Research (NIHR) announced that all new and existing studies supported through its Clinical Research Network would be paused to focus instead on COVID-19 research. You can read the full statement on the NIHR website.
The full HRA statement can be viewed here. If you have any queries mai in Research Development & Support.
Research Development & Support are also updating the following help page regularly for academics and researchers.
COVID-19 Pandemic: Public Health Implications in Nepal
Our editorial today in the Nepal Journal of Epidemiology highlights some of the key issues related to COVID-19 related to a low-income country such as Nepal [1]. There are various Public Health challenges to preventing the spread of COVID-19 in South Asia including Nepal. Learning from the COVID-19 outbreak in China, there will be slowdown of economic activity with damaged supply chains which impact upon the public health systems in Nepal. Moreover, there is limited coordination among different stakeholders in healthcare management with few policies in place for infection prevention and control, shortage of testing kits and medical supplies (shortages of masks, gloves), and poor reporting are major challenges to be tackled in case of the COVID-19.
All South Asian countries are vulnerable to a mass outbreak with high population density in cities which is challenging to create social distancing, made worse by generally poor hygiene and often low (health) literacy. Additionally, some COVID-19 cases remain asymptomatic; so it is difficult to predict the epidemic outbreak that may introduces further difficulty in diagnosis of newer cases. Finally, healthcare workers across the globe were infected at high rates during the MERS and SARS outbreaks, so Nepal has to initiate health workers’ training including simulation exercises to provide health staff with a clearer picture of the complexities and challenges associated with COVID-19 and containing potential outbreaks.
This editorial has a very different time span between submission and publication than the one highlighted last week on the BU Research Blog (see details here!). This COVID-19 editorial took exactly one month between submission and publication, the one mentioned last week took three-and-a-half years between submission and publication.
Prof. Edwin van Teijlingen
Reference:
- Asim, M., Sathian, B., van Teijlingen, E.R., Mekkodathil, A., Subramanya, S.H., Simkhada, P. (2020) COVID-19 Pandemic: Public Health Implications in Nepal, Nepal Journal of Epidemiology 10 (1): 817-820. https://www.nepjol.info/index.php/NJE/article/view/28269
COVID-19: health and social care research projects for educational purposes
The HRA have therefore decided stop reviewing applications for individual undergraduate and master’s student projects from now until further notice.
Please see this new guidance which relates to health and social care research projects conducted by undergraduate and master’s students: https://www.hra.nhs.uk/planning-and-improving-research/research-planning/student-research/
Three-and-a-half years to get published
Abstract
Junior colleagues or PhD students submitting their first manuscript often ask: “How long will it take before the editor comes back to me with a decision?” My stock answer is: “It depends!” It depends on the nature of the journal, the support available to the editor, how busy the editor is, or how difficult she finds it to allocate your paper to appropriate reviewers. Moreover, once sent out for review it depends how busy the reviewers are. I often have to remind my colleagues and student that academics are hardly ever paid for being a reviewer, and hence they do their reviewing over and above a usually heavy academic teaching and research load.
This short article highlights the case of one of our research papers which took four years from the day of submission to the journal to finally appearing in print. And, I hasten to say, it was not because the initially submitted manuscript was so bad that the authors had to make major changes and re-write or re-structure the whole paper. This blog highlights some of the unexpected hiccups in the process of getting an article published.
Introduction
On October the 5th 2016 Samridhi Pradhan, who worked for Green Tara Nepal in Kathmandu and who was Bournemouth University Visiting faculty at the time, emailed the then editor of an English-language journal based in Nepal with our manuscript. She wrote: “Respected Editor, I wish to submit a new manuscript entitled “Factors affecting the uptake of institutional delivery, antenatal and postnatal care services” in Nawalparasi district, Nepal in your esteemed Kathmandu University Medical Journal (KUMJ).” The editor wrote back the same day thanking us for our submission, and presenting us with a “manuscript ID o20161006250 for your valuable paper.…We will very soon go for initial screening and let you notify.” We expected the editor to send this manuscript out for review, then for us to get feedback a few months later, make some (minor) changes and have the paper published in 2017.
Then nothing happened for a year although we emailed and phoned the editor of KUMJ regularly. There seems to be a problem finding reviewers, and we offered to other potential reviewers. In December one of our co-authors Dr. Sharada Wasti informed our team that the KUMJ editor had suggested in a conversation with home that our paper would appear in the December volume of the journal. Dr. Waste emailed on 15 December 2017: “I will follow up and keep update with you by the end of this month.” KUMJ duly published Vol. 15 (4) labelled Oct.-Dec. 2017 but our paper was not included.
Four days later on December 19th 2017 we received an email for the editor with a set of minor comments and questions about the manuscript for reviewers. We made the requested amendments and resubmitted our paper on January 10th 2018. Months passed and KUMJ published two more issues in 2018 without our paper despite our team sending reminders regularly. Then on the last day of July 2018 Dr. Wasti emailed our team that he had had contact with the editor who had made it clear that: “This paper has been accepted” and it was supposed to come published in the last volume, which would have been the April-June 2018 issue Vol.16 (2). Dr. Wasti added that the editor had made it clear that although it was not covered, KUMJ “will publish in our upcoming volume.” This next issue Vol. 16 (3) again failed to publish our paper.
Then there was a long gap with KUMJ changing editors, correspondence being mislaid and manuscripts getting lost. Due to Dr. Wasti’s persistence in contacting the editors we received a request to update our paper with 2019 data in December 2019, nearly a year and a half after re-submission. A few days later that month we were informed that the journal was finally going to publish our paper. Another wo months passed before the editor email that say: “Congratulation, your paper has been finalized and selected for coming issue of KUMJ, paper has been uploaded in our website www.kumj.com.np.”
Some papers take longer to get published than others!
Our paper is now online first 3½ years after the initial submission. And to add insult to injury the journal is backdating the paper to appear with a publication date of September 2019, so it will be published in Vol.17(3) labelled July-Sept. 2019. This is, of course, an unusual story with a very long gap between first submission of a manuscript and the final appearance of the paper in print. It is unusual because the paper was only ever submitted to one journal. It is more likely that the publish process takes time because the first journal rejects the paper, the second paper also rejects your paper and then the subsequent paper asks for a set of alterations and changes. For example, van Teijlingen and Hundley (2002) outlined such process for one of their papers which took two years from initial submission to publication. However, this paper was submitted to five different journals in succession after having between rejected by the first four.
References:
- Pradhan, S., van Teijlingen E., Simkhada, P.P., Dhungel, A., Silwal R.C., Fanning P., Wasti, S.P. (2019) Factors Affecting the Uptake of Institutional Delivery, Antenatal and Postnatal Care in Nawalparasi District, Nepal, Kathmandu University Medical Journal http://www.kumj.com.np/issue/X/49-54.pdf (accepted).
- van Teijlingen, E., Hundley, V. (2002) Getting your paper to the right journal: a case study of an academic paper, Journal of Advanced Nursing 37(6): 506-511.
Latest CMMPH publication by Dr. Alison Taylor
 Congratulations to Dr. Alison Taylor in the Centre for Midwifery,Maternal & Perinatal Health (CMMPH) whose third PhD paper has just been accepted by the International Breastfeeding Journal. Alison’s paper ‘Commercialisation and commodification of breastfeeding: video diaries by first-time mothers’ reminds us that many of aspects of our lives are increasingly commercialised in post-modern society. Although breastfeeding is perhaps a late comer to this process in recent years, it too has seen significant commercialisation facilitated by social media and our obsession with celebrity culture.
Congratulations to Dr. Alison Taylor in the Centre for Midwifery,Maternal & Perinatal Health (CMMPH) whose third PhD paper has just been accepted by the International Breastfeeding Journal. Alison’s paper ‘Commercialisation and commodification of breastfeeding: video diaries by first-time mothers’ reminds us that many of aspects of our lives are increasingly commercialised in post-modern society. Although breastfeeding is perhaps a late comer to this process in recent years, it too has seen significant commercialisation facilitated by social media and our obsession with celebrity culture.
This paper explores how the commercialisation and commodification of breastfeeding impacts mothers’ experiences of breastfeeding. The paper highlights that women preparing for breastfeeding are exposed to increasing commercialisation. When things do not go to plan, women are even more exposed to commercial solutions. The impact of online marketing strategies fuelled their need for paraphernalia so that their dependence on such items became important aspects of their parenting and breastfeeding experiences. Dr. Taylor and her co-authors offer new insights into how advertising influenced mothers’ need for specialist equipment and services. Observing mothers in their video diaries, provided valuable insights into their parenting styles and how this affected their breastfeeding experience.

The International Breastfeeding Journal is an Open Access journal owned by Springer.
References:
- Taylor, A.M., van Teijlingen, E., Alexander, J., Ryan, K. (2020) Commercialisation and commodification of breastfeeding: video diaries by first-time mothers, International Breastfeeding Journal (accepted).
- Taylor A, van Teijlingen, E.,Ryan K, Alexander J (2019) ‘Scrutinised, judged & sabotaged’: A qualitative video diary study of first-time breastfeeding mothers, Midwifery 75: 16-23.
- Taylor, A.M., van Teijlingen, E., Alexander, J., Ryan, K. (2019) The therapeutic role of video diaries: A qualitative study involving breastfeeding mothers, Women & Birth 32(3):276-83. https://www.sciencedirect.com/science/article/pii/S1871519218300064
Nepal reproductive health paper published yesterday
Congratulations on the latest paper published yesterday by Dr. Preeti Mahato in the Centre for Midwifery, Maternal & Reproductive Health (CMMPH) and colleagues. This paper ‘Factors associated with contraceptive use in rural Nepal: Gender and decision-making’ [1], is freely available for the next 49 days through our personalized link: click here! 
This research paper in the journal Sexual and Reproductive Healthcare reports on a secondary analysis of pas a quantitative cross-sectional study in four villages of a hilly district in Nepal. This authors found that gender was associated with current/ever use of contraceptives but decision-making was not found associated with current/eve use of contraceptives. And, as perhaps was to be expected, socio-economic factors such as husband’s and wife’s education; and indicators showing sharing of childcare responsibilities were found to be associated with contraceptive use. the paper concludes that educational, health promotional and family planning programmes involving husbands are needed to promote use of contraceptives.
 Preeti’s co-authors are based at Dorset County Hospital in Dorchester, at CMMPH and at Singapore Clinical Research Institute/Duke-NUS Graduate Medical School, Singapore.
Preeti’s co-authors are based at Dorset County Hospital in Dorchester, at CMMPH and at Singapore Clinical Research Institute/Duke-NUS Graduate Medical School, Singapore.
Reference:
- Mahato, P., van Teijlingen, E., De Souza, N., Sheppard, Z. (2020) Factors associated with contraceptive use in rural Nepal: gender and decision-making, Sexual & Reproductive Healthcare 24: 100507 https://doi.org/10.1016/j.srhc.2020.100507
NIHR Grant Applications – seminar and support event – CANCELLED

24th March 2020
This event has been cancelled due to restrictions arising from COVID-19.
UKIERI Grant Success: Visit to India
The Departments of Psychology (SciTech), Midwifery and Health Sciences (HSS) from Bournemouth University and SSLA part of Symbiosis International (Deemed University) were successful in getting the United Kingdon India Education Research Initiative (UKIERI)  funding to support 10 UK Psychology Students and Staff to visit India. This initiative receives further support from Global Engagement Hub, Bournemouth University.
funding to support 10 UK Psychology Students and Staff to visit India. This initiative receives further support from Global Engagement Hub, Bournemouth University.
The Study in India Programme has been designed in collaboration with BU’s project partner university Symbiosis International in India, where this will be hosted. This exchange will offer a program of interactive lectures, workshops, research methods seminars, clinical experience observations, and relevant field visits.
Students will also contribute to research with Sheetal Astitva, which is a GCRF funded initiative aimed to improve mental health in rural India and Nepal. The lead researchers for this initiative are Prof. Edwin van-Teijlingen and Dr. Shanti Shanker.
Global Visiting Fellowships Scheme – Round 2
Funding available for researchers from partner institutions to become Global Visiting Fellows of BU
Fellows must be nominated by a member of academic staff at BU, and approved by the relevant Executive Dean.
Visit a drop-in session to find out more. Further info is also available in the guidelines.
16/03/2020 12:30 – 13:30 Lansdowne
To book , please contact Organisational Development. No authorisation is required.
If you have any queries, please contact ResearchFellowships@Bournemouth.ac.uk.
COVID-19 guidance for clinical researchers
The Health Research Authority have released guidance for clinical researchers, sponsors and sites with regard to COVID-19 (Coronavirus) – please read this guidance if you are currently conducting your project or are in the planning stages/set-up of the study (so you are aware of the current situation).
This guidance is subject to change and will be updated as and when required by the HRA.
You can read the guidance in the link provided above, but for convenience, these are the most likely situations BU clinical researchers may face. Please ensure that in planning amendments that these do not create additional burden to NHS staff or resources.
Amendments to existing studies impacted by wider COVID-19 response
- Example – Where changes to administrative arrangements are required to reduce burden or physical contact with sites – for example, changes to monitoring arrangements.
How this should be handled – these are non-substantial amendments that do not require HRA/HCRW approval or NHS R&D agreement. Where the study involves the NHS, they will be marked by the sponsor as a category C amendment not requiring assessment, and sent directly to the sites. The site should implement the amendment on the date specified by the sponsor.
- Example – Where changes are made to how or when patients are seen to avoid exposing patients or to reduce burden on clinical services – for example, changing site visits to phone calls or postal questionnaires.
How this should be handled – these are non-substantial amendments that do not require HRA/HCRW approval or NHS R&D agreement. The same procedure as the first example should be followed.
- Example – Where a temporary halt needs to be placed on some or all of the study, or the duration of the study needs to be extended.
How this should be handled – these are non-substantial amendments that do not require HRA/HCRW approval or NHS R&D agreement. The same procedure as above should be followed.
- Example – studies that need to be closed.
How this should be handled – for studies not involving provision of treatment to participants, a notification to the REC or study-wide review (for non-REC studies) should be provided, and an end of study report should subsequently be provided.
For any studies involving provision of treatment to participants, careful consideration should be given to post-study care. If this cannot be in line with the information provided in the participant-information sheet, a substantial amendment should be submitted.
To support sites in implementing the amendments it is important that:
- The changes and local implications are made clear
- Any changes to documentation are provided in tracked changes
- In England and Wales All correspondence to sites should be copied to R&D/I department and the PI and delivery teams
- Where indicated above, the sponsor should include the category and confirm that no assessment is required.
There may be some instances in which the site may raise issues or changes that need to be made. If they do, please inform the Sponsor as soon as possible.
What to do next
If you think that you may need to implement any changes or amendments to your study due to COVID-19 please get in touch with us as soon as possible. If you have any concerns or queries then please also get in touch to discuss these.













 New CMWH paper on maternity care
New CMWH paper on maternity care From Sustainable Research to Sustainable Research Lives: Reflections from the SPROUT Network Event
From Sustainable Research to Sustainable Research Lives: Reflections from the SPROUT Network Event REF Code of Practice consultation is open!
REF Code of Practice consultation is open! ECR Funding Open Call: Research Culture & Community Grant – Apply now
ECR Funding Open Call: Research Culture & Community Grant – Apply now ECR Funding Open Call: Research Culture & Community Grant – Application Deadline Friday 12 December
ECR Funding Open Call: Research Culture & Community Grant – Application Deadline Friday 12 December MSCA Postdoctoral Fellowships 2025 Call
MSCA Postdoctoral Fellowships 2025 Call ERC Advanced Grant 2025 Webinar
ERC Advanced Grant 2025 Webinar Update on UKRO services
Update on UKRO services European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease
European research project exploring use of ‘virtual twins’ to better manage metabolic associated fatty liver disease